How Do You Like Your Acetal: Homopolymer or Copolymer?
Acetal materials have been a commercial option for more than 50 years.
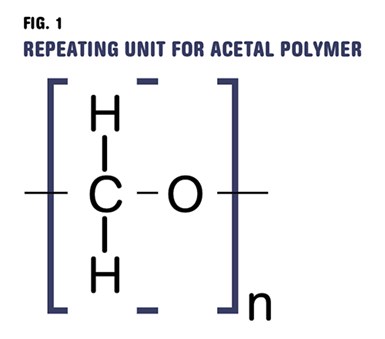
Polyoxymethylene (POM) is so named because that chemical group is the repeating unit in the polymer chain.
Acetal materials have been a commercial option for more than 50 years. Because of the lack of standardized nomenclature in our industry, these materials are often referred to as polyacetal (after the name of the original terminating group on the chain), polyformaldehyde (formaldehyde being the starting material for the polymerization), and polyoxymethylene (POM). This last one seems to have gained some traction, at least as the official abbreviation, and the name arises from the chemical group shown in Fig. 1, which represents the repeating unit in the polymer chain.
Acetals were known at the laboratory level as far back as the 1920s, but it took until the mid-1950s to solve the problems associated with the thermal stability of the material. Some who process the materials would maintain that this problem is still unresolved, and almost everyone who has been in the plastics processing business for a while has their favorite story about hoppers being ejected from their moorings on the machine or a nozzle suddenly turning into a projectile as large amounts of formaldehyde gas were generated by an overheated mass of acetal polymer. But in fact, the process stability of the material is much better today than it was when the first materials were introduced.
Those first materials were homopolymers, meaning that every repeating unit in each individual polymer chain looked like the one in Fig. 1. A few years later, acetal copolymers were created and commercialized, and ever since then molders and end users have had a choice between the two families. But the reason for selecting homopolymer or copolymer has not always been clear to those making the decision.
Processors have tended to favor copolymers because they provide a wider processing window. Since acetals are highly crystalline, they must be heated above their melting point to be processed. Homopolymers possess a greater degree of regularity in their structure; therefore they are more crystalline than their copolymer counterparts. The other monomer used to make the copolymers has a longer hydrocarbon linkage that increases the spacing between the oxygen atoms in the polymer chain. These oxygen atoms are the points of greatest susceptibility for thermal and oxidative degradation, so the fewer of these that are present, the more resistant the material will be to degradation.
But creating that reduced level of regularity also reduces the crystallinity. This is good for the processor because it results in a melting point for the copolymer that is about 10°C lower than that of the homopolymer. Thus the copolymers potentially can be processed at a lower melt temperature. In addition, the greater spacing of the vulnerable oxygen atoms increases the material’s tolerance for higher melt temperatures. So copolymers have a wider processing window because of advantages on both ends of the thermal spectrum.
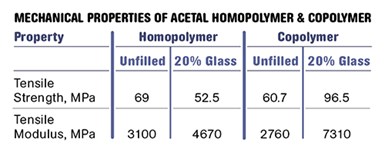
But the lower degree of crystallinity in the copolymers brings a reduction in strength and modulus as well as the upper limit on short-term use-temperature conditions. The tensile strength of a typical homopolymer is about 15% higher than that of the corresponding copolymer and the modulus is nearly 13% greater. The higher level of crystallinity also translates into improved bearing properties as well better fatigue and creep resistance. Figure 2 shows plots of elastic modulus as a function of temperature for medium-molecular-weight unfilled grades of homopolymer and copolymer.
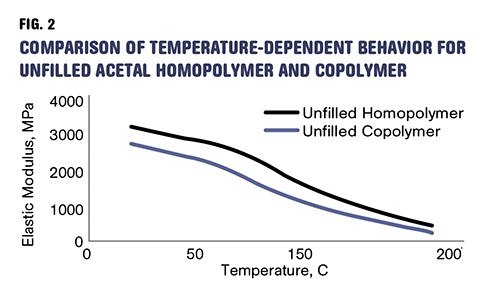
As shown in medium-MW acetals, unfilled homopolymer is stiffer and stronger than unfilled copolymer, across a very broad temperature range.
But while the short-term heat resistance of the homopolymer is better, the presence of the additional hydrocarbon linkages in the copolymer backbone provides for improved resistance to oxidation. This translates into better property retention after long-term exposure to elevated temperatures, plus improved chemical resistance—particularly in acidic and alkaline environments.
One diagnostic test for determining if an acetal part was molded from a homopolymer or a copolymer is called the TEA. TEA stands for triethanolamine, a very strong base. If a sample of a homopolymer is placed in TEA at an elevated temperature it dissolves fairly rapidly while the copolymer does not. One very important application where this difference in chemical resistance becomes a factor is in exposure to hot water, especially if the water is chlorinated. And any environment where long-term exposure to elevated temperatures is a factor will produce an eventual narrowing of the difference in mechanical properties, because the copolymer survives the exposure better than the homopolymer.
There is an interesting and often overlooked difference between the two materials. It can be observed by examining the strength and modulus of the unfilled and glass-filled grades of the material. We have already mentioned that the unfilled homopolymer is stiffer and stronger than the unfilled copolymer. Figure 2 shows the elastic modulus as a function of temperature for the two materials and confirms that this is true not only at room temperature but across a very broad temperature range that extends all the way to the melting point of the materials.
However, if we examine the data sheets for 20% glass-fiber filled grades of these two materials we see something very unusual. The strength of the copolymer increases by 60% and the modulus is 2.5 times higher when glass fiber is incorporated. While the modulus of the homopolymer also increases, the improvement is more modest, resulting in a situation where the copolymer with glass is actually stiffer than the filled homopolymer. In addition, the tensile strength of the homopolymer actually declines with the addition of glass.
The accompanying table summarizes these changes as they appear in the literature, and Fig. 3 shows the modulus vs. temperature behavior for the two glass-filled materials. The copolymer is now the stiffer material and can be expected to outperform the homopolymer even in the areas of creep and fatigue, where the homopolymer held the advantage in the unfilled grades.
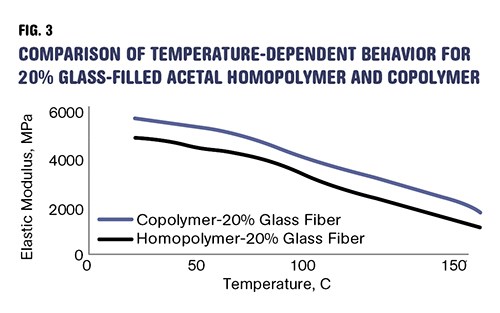
Adding glass fiber makes acetal copolymer stiffer than homopolymer. Reinforced copolymer can be expected to outperform homopolymer in the areas of creep and fatigue, where the homopolymer held the advantage in the unfilled grades.
The reason for this behavior has to do with the manner in which the glass interacts with the polymer. In most polymers glass fiber acts as a reinforcement: It adds strength as well as stiffness. In order for this to occur a good bond must be formed between the polymer and the glass fiber. Some polymers like nylon have a chemistry that naturally bonds well to glass and the resulting property improvement reflects this. Other materials such as polypropylene do not have a natural affinity for glass and therefore some modification of the polymer is required in order to achieve this bond.
This modification is known as coupling or chemical coupling and it has been employed in acetal copolymers to create the observed improvements in mechanical properties. However, the chemistry of the homopolymer makes coupling nearly impossible. Consequently, the glass fiber acts more like a filler than a reinforcement, adding stiffness but not strength. This is an important distinction when evaluating these two material families in the filled state.
One more thing of interest that applies equally to both families is the inability with today’s technology to impart flame-retardant properties to acetals. The traditional route of using halogenated FR systems is not an option with acetals because of the violent reaction that these polymers undergo with any chlorinated or brominated substances. Polymer chemists are a very clever group and there is nothing to say that at some point in the future someone will not develop an FR acetal. But so far, we all have to be content with a UL rating of HB for all acetal materials.
ABOUT THE AUTHOR: Michael Sepe is an independent materials and processing consultant based in Sedona, Ariz., with clients throughout North America, Europe, and Asia. He has more than 45 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting, and failure analysis. Contact: (928) 203-0408 • mike@thematerialanalyst.com
Related Content
Back to Basics on Mold Venting (Part 1)
Here’s what you need to know to improve the quality of your parts and to protect your molds.
Read MoreUnderstanding Melting in Single-Screw Extruders
You can better visualize the melting process by “flipping” the observation point so the barrel appears to be turning clockwise around a stationary screw.
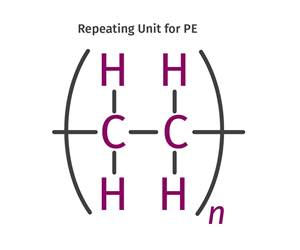
Read MoreThe Fundamentals of Polyethylene – Part 2: Density and Molecular Weight
PE properties can be adjusted either by changing the molecular weight or by altering the density. While this increases the possible combinations of properties, it also requires that the specification for the material be precise.
Read MoreInjection Molding: Focus on these Seven Areas to Set a Preventive Maintenance Schedule
Performing fundamental maintenance inspections frequently assures press longevity and process stability. Here’s a checklist to help you stay on top of seven key systems.
Read MoreRead Next
For PLASTICS' CEO Seaholm, NPE to Shine Light on Sustainability Successes
With advocacy, communication and sustainability as three main pillars, Seaholm leads a trade association to NPE that ‘is more active today than we have ever been.’
Read MoreSee Recyclers Close the Loop on Trade Show Production Scrap at NPE2024
A collaboration between show organizer PLASTICS, recycler CPR and size reduction experts WEIMA and Conair recovered and recycled all production scrap at NPE2024.
Read MoreBeyond Prototypes: 8 Ways the Plastics Industry Is Using 3D Printing
Plastics processors are finding applications for 3D printing around the plant and across the supply chain. Here are 8 examples to look for at NPE2024.
Read More
.jpg;width=70;height=70;mode=crop)