Acetal Resin Series for Medical Applications
Polyplastics’ Duracon POM PM series launched globally.
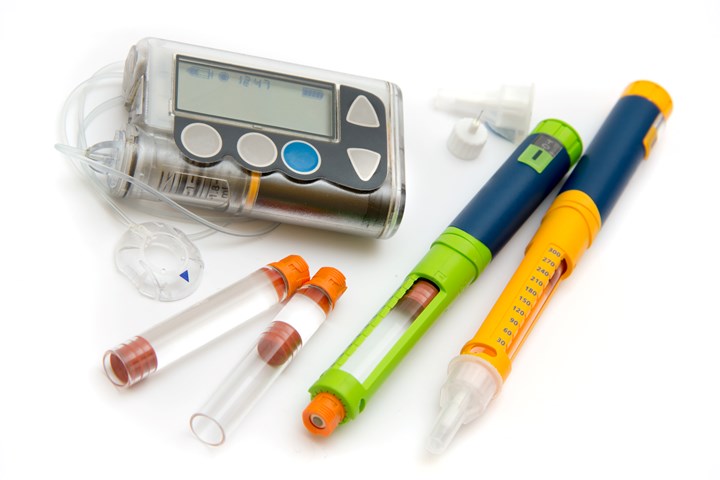
Commercial availability of a new series of acetal resins for medical applications from the Polyplastics Group has been launched. The company will supply Duracon POM PM (Polyoxymethylene/Acetal) series to all global regions including Asia such as China and India, and Europe and the U.S. Polyplastics has served the medical and healthcare field for decades with its high-purity Topas COC (cyclic olefin copolymer). As the world’s leading POM manufacturer, Polyplastics now adds the PM Series to its medical grade portfolio and plans to expand material supply to the medical and healthcare market.
Polyplastics’ two acetal grades include a standard viscosity grade, PM09S01N, which delivers the reliable mechanical properties and moldability expected from POM. In addition, a high-flow grade, PM27S01N, will enable wall-thinning, miniaturization, and lightweighting of medical devices that are becoming more complex and highly functional. Both grades meet medical device manufacturers’ key requirements and are targeted for a wide range of applications such as drug contact and delivery. Both also offer global market regulatory compliance including ISO 10993 and USP Class VI biocompatibility/cytotoxicity, FDA Drug Master File (DMF) and Device Master File (MAF), and EU 10/2011 and FDA food contact 21 CFR 177.2470. The materials can be sterilized under hot steam and ethylene oxide (EtO) sterilization conditions.
Related Content
-
Understanding the ‘Science’ of Color
And as with all sciences, there are fundamentals that must be considered to do color right. Here’s a helpful start.
-
Commodity Resin Prices Flat to Lower
Major price correction looms for PP, and lower prices are projected for PE, PS, PVC and PET.
-
Understanding Strain-Rate Sensitivity In Polymers
Material behavior is fundamentally determined by the equivalence of time and temperature. But that principle tends to be lost on processors and designers. Here’s some guidance.













