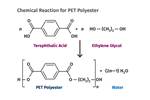
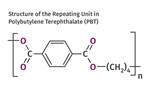
Tracing the History of Polymeric Materials: Polyesters
Beyond PET, PBT and their analogues, development of polyester chemistry led to unsaturated thermosetting resins, copolyester thermoplastic elastomers, liquid-crystal polymers and, most recently, biopolymers.
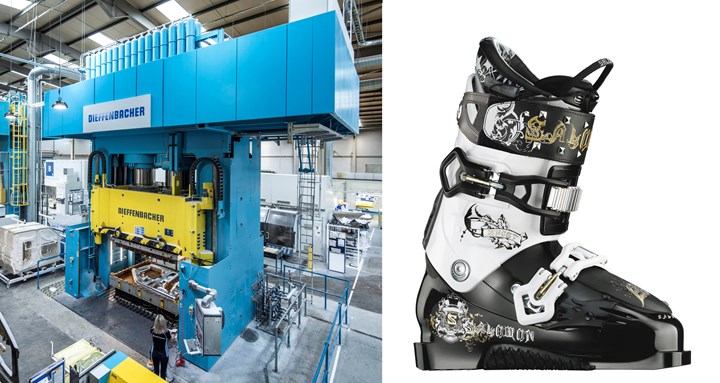
Polyester technologies have proliferated from thermoset unsaturated polyester, used in fiberglass composites like SMC (left), to copolyester TP elastomers like DuPont’s Hytrel. (Photos: Dieffenbacher and DuPont)
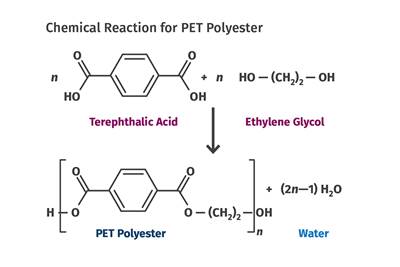
The versatility of polyester chemistry has been demonstrated over the nine decades since its original introduction. Much of the plastics industry today is focused on thermoplastic materials. However, one of the most important polyester chemistries is unsaturated polyesters and vinyl esters. These materials are created using chemical reactions similar to those already discussed for PET and PBT. However, in this case, a difunctional alcohol such as propylene glycol is reacted with a combination of saturated and unsaturated organic acids or anhydrides with reactive groups that extend in more than two directions. This chemistry allows for the process of crosslinking using a material like styrene monomer or vinyl acetate to create a thermosetting material.
These chemistries were discovered by Wallace Carothers and his team at DuPont in the early 1930s at the same time that they were investigating the early polyester fiber chemistry. The original polymer developed by Carothers used fumaric acid, maleic anhydride, and ethylene glycol. The reaction required a relatively high temperature and resulted in a material of very high viscosity that was difficult to work with and cured very slowly. Like the aliphatic polyesters that DuPont developed at about this same time, these crosslinked versions were not commercialized. But in 1933, Carlton Ellis, an inventor and pioneer in organic chemistry, introduced the vinyl monomer styrene into the process as a crosslinking agent. This resulted in a lower-viscosity resin to which glass fibers could be added prior to curing. Cure rates also increased dramatically with this innovation.
This allowed the production of very large structures with good strength and stiffness. Unfortunately, at the time, styrene was still a specialty chemical with a very high price tag, so commercialization was slow. But with the start of World War II the need for synthetic rubber became a priority and the government financed the construction of multiple plants to produce styrene-butadiene rubber (SBR), making styrene more available and dropping the price to a point where production of unsaturated polyesters at a reasonable cost became feasible. One of the important characteristics of unsaturated polyesters is its transparency to radar, an obvious advantage in wartime. Commercialization is generally dated at 1939, a couple of years before the thermoplastic variety was introduced.
These products, often referred to as “fiberglass,” are still used today to make large structures for the marine, building and construction, and automotive industries. One of the iconic models of the American automotive industry, the Corvette, introduced in 1953, makes extensive use of these materials in their exterior body panels. Ellis was awarded the first U.S. patent for unsaturated polyester in 1933 and made additional improvements in the product up to the time of his death in 1941 by varying the chemistry of the organic acids, anhydrides, alcohols, and crosslinking agents. He was inducted into the Plastics Hall of Fame in 1974. Today these polymers are used to produce materials known as bulk molding compounds (BMC) and sheet molding compounds (SMC) that can be compression, transfer, or injection molded into products that remain crucial for the automotive, appliance, and electrical industries.
Unsaturated polyester can provide a combination of excellent electrical properties and flame suppression that no thermoplastic has been able to replicate, while delivering good mechanical performance and resistance to elevated temperatures. Polymer concrete is also based on unsaturated polyester. A closely related chemistry, polydicyclopentadiene (PDCPD), is also used in larger structures processed through a variety of methods such as reaction injection molding (RIM), resin transfer molding (RTM) and compression molding.
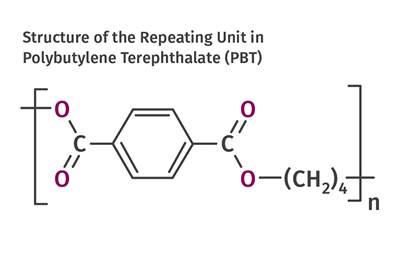
In 1970, PBT polyester was first incorporated into a new set of compounds that had elastomeric properties.
Ellis also has an intersection with the development of the cellulose acetate lacquers created during World War I that we discussed in Part 7 of this series (May ’21). These lacquers provided the wood in the airplanes of that time with resistance to moisture and fire. The lacquers were produced by dissolving cellulose acetate polymer in acetone, a chemical that was in limited supply at the time. In 1916, Ellis developed a more efficient method for making isopropanol, which is then converted to acetone. This broke the bottleneck in producing these materials.
In 1970, PBT polyester was incorporated into a new set of compounds that had elastomeric properties. Commercially referred to as copolyester elastomers, these materials consisted of PBT and segments of a longer-chain diol that formed a group referred to as poly tetramethylene oxide (PTMO). The PBT crystallized to provide the hard structure while the PTMO remained amorphous, providing the flexibility and good low-temperature ductility. The properties could be varied by altering the ratio of PBT and PTMO to produce a range of TP elastomers with different thermal and mechanical properties and surface hardness. These were first introduced by DuPont under the Hytrel trade name and today have several competitors, including grades from Celanese and DSM.
The first melt-processable LCPs appeared in the 1980s, suited to uses like electronic connectors. (Photo: Celanese)
Another fascinating variant on polyester chemistry is liquid-crystal polymers (LCPs). Liquid-crystal forms in small molecules had been studied since the 19th century, but the first well-known commercial example of a liquid-crystal polymer was Kevlar, invented at DuPont in 1965 by chemist Stephanie Kwolek. This was followed in 1967 by Nomex.
Both of these materials are based on polyamide chemistry and make extensive used of an aromatic backbone structure. While exhibiting remarkable properties, they have melting points that exceed the degradation temperature of the polymer and therefore can not be melt processed. These are referred to as lyotropic LCPs. Thermotropic LCPs were commercially introduced around 1980 and are based primarily on polyester chemistry. The principal constituent is p-hydroxybenzoic acid. However, homopolymers of this material have melting points above 500 C (932 F), which presents obvious problems for an industry running machines with maximum achievable barrel temperatures of 427 C (800 F).
The first melt-processable LCP product line came from the unlikely source of Dart Industries, which at the time owned Tupperware. The first commercial grades had very high melting points, close to 420 C (788 F) and Dart targeted high-heat cookware, called Ultra 21, as an addition to the Tupperware line. In the late 1980s I owned several of these items and surprised a friend of mine from GE Plastics when I served lasagna cooked in a pan made from this material. The items were expensive compared with the traditional polypropylene Tupperware products and the surfaces had to be treated carefully or they scratched easily. But they were a novelty that illustrated the potential of LCP to operate at high temperatures.
Following the history of TP polyesters, newer biopolymers may need to evolve from aliphatic to aromatic chemistries in order to thrive.
A more concerted effort to develop a functional line of LCP products was taken on by Celanese in the mid-1980s with the Vectra line that is still very active today. By introducing other comonomers, the heat resistance of the material could be tailored to hit certain targets, and this is what primarily distinguishes different grades of LCP today. In addition to their excellent thermal properties, LCP materials exhibit very low melt viscosity, enabling production of parts with very thin walls. They also allow for very fast cycle times since the energy barrier between the solid and the liquid state is very small. With the appropriate fiber reinforcement, they can provide exceptional strength and stiffness, although weld-line integrity is a notable problem. Interestingly, DuPont also developed an LCP line somewhat later under the trade name Zenite, but has since sold that line to Celanese.

Aliphatic constituents contribute rather modest performance properties to the current crop of biopolymers like PLA, PHA and others. Future development may benefit from incorporation of aromatic chemistries. (Photo: Milliken)
One other intersection with polyester chemistry is the current offerings in biopolymers. Most biopolymers are derived from starches, sugars, or cellulose. Many of the resulting polymers are polyesters. But at this point, the monomers being used are aliphatic. Therefore, the resulting materials have properties that are comparable to the original aliphatic polyesters developed in the 1930s, which were abandoned due to poor thermal and mechanical performance. This indicates that future development of materials from renewable sources may require aromatic chemistries in order to be considered viable.
Throughout our discussion of nylons and polyesters, the same players keep showing up, and an examination of the chemistry shows that there are many similarities between these two polymers that result in overlaps in applications. This presents challenges in the material-selection process. In our next installment we will pause our historical treatment to discuss the relative merits of selecting a polyester or a nylon polymer for an application and highlight some notable instances where an industry successfully converted from one to the other.
Related Content
Fundamentals of Polyethylene – Part 6: PE Performance
Don’t assume you know everything there is to know about PE because it’s been around so long. Here is yet another example of how the performance of PE is influenced by molecular weight and density.
Read MoreThe Effects of Time on Polymers
Last month we briefly discussed the influence of temperature on the mechanical properties of polymers and reviewed some of the structural considerations that govern these effects.
Read MoreUnderstanding Melting in Single-Screw Extruders
You can better visualize the melting process by “flipping” the observation point so the barrel appears to be turning clockwise around a stationary screw.
Read MoreWhere and How to Vent Injection Molds: Part 3
Questioning several “rules of thumb” about venting injection molds.
Read MoreRead Next
Tracing the History of Polymeric Materials: PET
How PET evolved from a material for fibers and fabrics to a force in packaging.
Read MoreTracing the History of Polymeric Materials: PBT
The slow crystallization of PET polyester made it a poor option for processes like injection molding. This led to the development of more molder-friendly options such as PBT.
Read MoreTracing the History of Polymeric Materials: How Nylons & Polyesters Connected
The history of nylons and polyesters are intertwined, and it takes some knowledge of chemistry to understand why.
Read More
.jpg;width=70;height=70;mode=crop)