Clean-Room LSR Molding Cell For New Approach to HIV/AIDS Prevention
Injection press, metering system, and mold are engineered for medical molding of drug-impregnated LSR.
Although it’s not so much in the headlines these days, HIV/AIDS is still one of the most dangerous diseases on the planet. The World Health Organization says that 34 million people worldwide were living with HIV/AIDS in 2011 and 1.7 million died of AIDS-related illnesses.
A new weapon in the fight against this disease is in Stage III clinical testing by the U.S. FDA. It deploys an antiviral drug mixed into two-component liquid silicone rubber (LSR), which is injection molded into a drug-releasing vaginal ring. An optimized mixing procedure was tailored for this particular drug, and the machine, metering system, and mold were all designed to operate with a clean-room cell that meets the standards of ISO 13485 and FDA’s Good Manufacturing Practices (GMP) guidelines and also exceeds Six Sigma quality levels.
The “drug-releasing polymer vaginal ring” is being developed by QPharma AB of Malmö, Sweden, which is both the OEM and the molder of the product. A trial production system was built using an all-electric injection machine from Arburg of Germany (U.S. office in Newington, Conn.), LSR metering system from 2KM of Germany (North American office in Parry Sound, Ont.) and mold from Rico Elastomere of Austria.
PREMIXING THE DRUG IN LSR
The gray-white LSR vaginal ring weighs 8 g and contains 25 mg of the microbiocide Dapivirine, as well as 30% silica in both A and B components. To meet the product’s quality standard, the drug dosage must stay between 22.5 and 27.5 mg. The active ingredient is a micropowder that does not dissolve in the two-component, platinum-curing LSR. To ensure uniform distribution of the HIV-specific antiviral in the ring, QPharma uses noncontacting, nondestructive Raman spectroscopic analysis of each molded part.
Initial testing ruled out premixing the drug with silicone oil, added as a third stream in molding the LSR. The active ingredient separated from the oil, resulting in lower concentrations in the molded rings. Best results were obtained by premixing the micropowder with silicone oil and adding that mix to both LSR components in equal parts before they are merged in the 2KM metering system. Process stability was twice as good as with the three-stream system, and drug distribution in the rings was more uniform.
PRODUCTION FOR CLINICAL TRIALS
The vaginal ring has an O.D. of 56 mm and thickness of 7.7 mm. It is molded in 16 cavities at 100 to 200 C. The non-optimized cycle was between 1 and 2 min. Total shot is 125 cm³, injection time is 6.5 sec, and holding pressure is 250 bar (3628 psi).
Trials determined that the percentage of microbiocide was not affected by the injection molding process. Shot volume and holding pressure were the key quality-determining factors, essential for maintaining both the I.D. of the ring and correct weight. Processing parameters like time had very little influence on part quality. Therefore, it was possible to scale back the process settings to prevent overfilling.
The clean-room manufacturing cell was built around an Arburg Allrounder 520 A electric press with 150-metric-ton clamp and 232-g injection unit. The machine was designed not only for LSR processing but also to meet strict hygienic standards for medical molding. Its clean-room design included the following:
• Clamp section in stainless steel;
• Encapsulated toggle levers and covered ejector area;
• FDA/NSF-approved lubrication;
• Fully contained water distribution;
• Sleeves and rapid-release fittings of stainless steel;
• Nickel-coated clamp platens.
A self-contained clean-room cell surrounds the clamp area and automation system. Clean-air modules with ionization are ISO class 3.
The cell includes a six-axis robot, also adapted to clean-room standards and fitted with a special gripper and conveyor belt. Demolding via robot instead of compressed air minimizes contamination in the clean room.
Inline 100% QC is accomplished with Raman spectroscopic analysis and optical measurement of parts. Tolerance of the camera system is ±0.025 mm.
Supply pressure of the 2KM metering system—located outside the clean-room enclosure—was controlled graphically, and the flow rate and temperature of the mold cooling-water supply were also monitored.
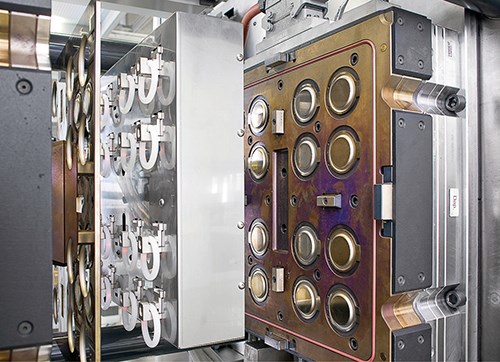
An essential component of the system is a tool with a perfect finish that holds very narrow tolerances over a long time. The Rico 16-cavity tool utilizes the company’s standard approach for LSR, which uses cold-runner (i.e., runnerless) direct injection into each cavity, thereby eliminating sprue and runner scrap, ensuring maximum accuracy, and facilitating high-speed, automated production with less robotics.
Coated insulation plates suitable for clean rooms avoid particle contamination of ambient air. Rico insulates its tools to minimize heated tool volume and limit air-conditioning requirements for the clean room.
Rico designed the mold to avoid external drill holes and indentations in order to facilitate tool cleaning and prevent particle accumulation. Other mold features include:
• Stainless-steel connections for water, air, etc.;
• Maintenance-free, lubrication-free mold guides;
• Electric mold heating to eliminate the need for oil or other heating medium;
• Use of vacuum to reduce venting and minimize burr formation;
• Cavity components with no coatings.
Due to the tight product tolerances, the tool had to be very carefully balanced to eliminate any inconsistencies between the 16 cavities. O.D. of the LSR rings ranged from 55.843 to 55.988 mm, a deviation of only 0.145 mm. Weight consistency was also critical—maximum deviation between cavities was only 20 mg for the 8-g parts. Rico says the results go “well beyond the requirements of Six Sigma.”
Related Content
What to Look for in High-Speed Automation for Pipette Production
Automation is a must-have for molders of pipettes. Make sure your supplier provides assurances of throughput and output, manpower utilization, floor space consumption and payback period.
Read MoreImpacts of Auto’s Switch to Sustainability
Of all the trends you can see at NPE2024, this one is BIG. Not only is the auto industry transitioning to electrification but there are concerted efforts to modify the materials used, especially polymers, for interior applications.
Read MoreFor Extrusion and Injection-Blow Molders, Numerous Upgrades in Machines and Services
Uniloy is revising its machinery lines across the board and strengthening after-sales services in tooling maintenance, spare parts and tech service.
Read More‘Monomaterial’ Trend in Packaging and Beyond Will Only Thrive
In terms of sustainability measures, monomaterial structures are already making good headway and will evolve even further.
Read MoreRead Next
Getting Into LSR: Part II--Chosing an Injection Machine
The criteria for selecting a liquid silicone rubber injection molding machine are very similar to selecting a machine for standard thermoplastic injection molding, with several key differences.
Read MoreGetting Started in LSR: Understanding the Materials, Part I
Liquid silicone rubber (LSR) injection molding is a long established process but it is enjoying an upsurge in interest for medical, automotive, infant care, and general industrial applications.
Read MoreLSR Part III-- Choosing a Mixing/Metering System
Liquid silicone rubber (LSR ) is a two component reactive chemical with a viscous, paste-like consistency.
Read More