PBT & PET Polyester: Part 2 The Performance Factor
Materials Know How
All things being equal, PET will outperform PBT mechanically and thermally. But the processor must dry the material properly and must understand the importance of mold temperature in achieving a degree of crystallinity that allows the natural advantages of the polymer to be realized.
Last month, we discussed the chemistry of PBT and PET polyesters and the thermal properties that arise from those chemistries.
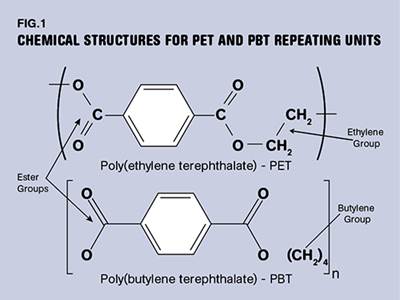
We also pointed out that PET can be provided in both amorphous and semi-crystalline forms while PBT under normal circumstances is always semi-crystalline. In order for PET to crystallize in the relatively short time frames of processes like injection molding, nucleating agents and fillers or reinforcements are added. Therefore, as we start looking at the performance of these two families of polyesters, we must compare apples to apples—reinforced or filled grades of the semi-crystalline varieties from both families.
IMPORTANCE OF GLASS TRANSITION
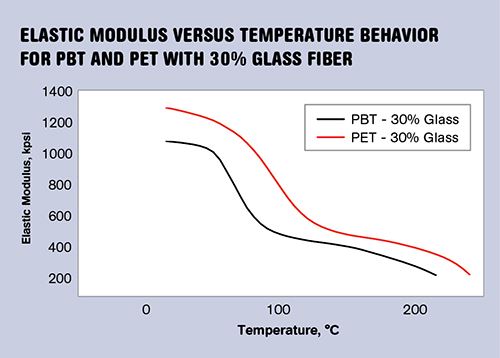
The graph to the right shows a comparison of 30% glass-fiber reinforced PET and PBT polyesters. The room-temperature performance reflects the differences that can also be observed on data sheets—i.e., that PET is about 20% stiffer. But the graph provides a more complete picture that shows how the superior properties of PET persist across the temperature range from room temperature to the point where the two materials begin to melt. Both materials undergo a step change in modulus that reduces the stiffness of the materials by a little more than 50%.
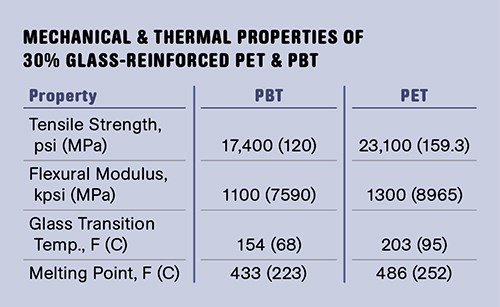
This is the glass transition and it is a very important property for designers to be aware of, since it lowers the expectations for mechanical performance at elevated temperatures. The glass-transition temperature (Tg) for PET is nearly 30° C higher than that of PBT, an offset that is essentially the same as that reflected in the melting points. The table at right captures some mechanical and thermal properties that distinguish the two polymers from each other. In addition to greater stiffness and higher glass-transition and melting temperatures, PET is 35% stronger at room temperature.
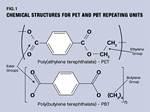
This difference in performance is a result of the closer spacing of the ester groups and aromatic rings in the PET chain. These constituents lend stiffness that results in the higher Tg and create the polar bonds that provide for a higher melting point. The ethylene and butylene linkages in between provide for molecular mobility that is important for achieving crystallinity as the material cools. The Tg is also an important consideration for processors, since achieving an optimal degree of crystallinity in the molded part requires that the polymer be kept above its Tg long enough to allow crystallization to take place.
Crystallization occurs when the polymer is cool enough to solidify but still warm enough to possess molecular motion that allows polymer chains to organize. These organized regions are the crystals in the semi-crystalline material. They define many properties including creep resistance, fatigue resistance, and retention of properties at elevated temperatures. A high Tg is a double-edged sword. It provides improved mechanical performance over a broader temperature range, but it also means that the processor needs to pay more attention to the cooling rate of the material.
This becomes especially important in thin-walled parts where the mold temperature has a more direct effect on the actual temperature of the polymer. Rapid cooling can result in quenching, and this reduces the degree of crystallinity achieved in the final product. The longer butylene linkages in PBT provide increased molecular mobility, which produces a lower Tg. This is a disadvantage for the end user but makes life easier for the processor. In injection molding, a mold temperature that exceeds 155-160 F will produce a stable and optimal structure in PBT. In PET the lowest mold temperature that can be practically employed while still achieving optimal mechanical performance is 200-215 F, depending upon the part geometry.
And this assumes a very efficient nucleating system. Not all PET polyesters are created equal in this respect. Some PETs crystallize efficiently at these mold temperatures while others require mold temperatures as high as 250 F. This presents challenges for some processors.
Choose your PET supplier carefully with respect to this parameter. I once worked with a molder who changed suppliers to save a few cents per pound but lost a great deal of mechanical performance at elevated temperatures because the mold temperatures it employed did not produce full crystallization in the less-expensive resin. They did not find this out until parts began to fail fatigue tests at 85 C.
The good news is that the melt viscosity of semi-crystalline grades of PET polyester tends to be lower than PBT at the same level of reinforcement. This means that more intricate shapes can be molded, thinner walls can be filled, and a more resin-rich finish can be attained in highly filled PET compounds. If a PBT polyester is sold as a material that achieves an improved surface finish, the chances are pretty good that it is blend of PBT and PET.
WHAT ABOUT DRYING?
We would be remiss if we did not discuss the issue of moisture content and drying. Polyesters are susceptible to hydrolysis, a chemical process that involves the breaking of chemical bonds through a reaction with water. If PBT or PET is heated above the melting point in the presence of excess moisture, the polymer chains break. This reduces the molecular weight of the material, which in turn reduces mechanical performance. This even happens in the solid state, but the process takes weeks or months because of the lower temperatures. In the molten state, it happens in a few minutes. But the reaction rate is much faster in PET and requires less moisture.
Twenty to 30 years ago the processing literature stated that a maximum moisture content of 400 ppm (0.04%) was acceptable for PBT while the maximum allowable moisture content for PET was 200 ppm (0.02%). Some of the newer literature for PBT has now also adopted the 200 ppm guideline. However, studies have shown that in practice the stated limitation for PET is to be taken very seriously, while PBT can survive processing at levels as high as 750-1000 ppm without experiencing any significant level of degradation, as long as the melt temperature is tightly controlled.
This difference in behavior creates the perception in the industry that PET is harder to dry.
However, studies have shown that moisture reduction rates in equivalent desiccant dryers are essentially the same and moisture uptake rates after removal from the dryer are also similar. The difference is that if you neglect to properly dry PET, the feedback in the form of reduced performance is immediate and often catastrophic. With PBT the consequences are often not noticeable until things really get out of hand. So when a processor relates difficulties with brittle PET when it never experienced the same problems with PBT, the material often gets the blame when in reality it is the processor who is responsible.
All things being equal, PET will outperform PBT mechanically and thermally. But in order to achieve this superior performance the processor must dry the material properly and must understand the importance of mold temperature in achieving a degree of crystallinity that allows the natural advantages of the polymer to be realized.
About the Author
Michael Sepe is an independent materials and processing consultant based in Sedona, Ariz. with clients throughout North America, Europe, and Asia. He has more than 35 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting, and failure analysis. Contact: (928) 203-0408 • mike@thematerialanalyst.com.
Related Content
Brewer Chooses Quick-Change Flexibility to Blow Wide Range of PET Beer Bottles
Beermaster Brewery found a “universal” stretch-blow machine from PET Technologies enables multiple changes per day among four sizes of beer bottles.
Read MoreHow to Decrease the Extrudate Temperature in Single-Screw Extruders
In many cases, decreasing the discharge temperature will improve product quality and perhaps even boost rate. Here are ways to do it.
Read MoreHow to Effectively Reduce Costs with Smart Auxiliaries Technology
As drying, blending and conveying technologies grow more sophisticated, they offer processors great opportunities to reduce cost through better energy efficiency, smaller equipment footprints, reduced scrap and quicker changeovers. Increased throughput and better utilization of primary processing equipment and manpower are the results.
Read MoreWhat You Need to Know About Roll-Cooling Design
Cooling rolls might not look like high-tech machines, but the fact is there is a surprising amount of technology involved in their design, manufacture and use. Here’s what you need to know.
Read MoreRead Next
PBT and PET Polyester: The Difference Crystallinity Makes
To properly understand the differences in performance between PET and PBT we need to compare apples to apples—the semi-crystalline forms of each polymer.
Read MorePeople 4.0 – How to Get Buy-In from Your Staff for Industry 4.0 Systems
Implementing a production monitoring system as the foundation of a ‘smart factory’ is about integrating people with new technology as much as it is about integrating machines and computers. Here are tips from a company that has gone through the process.
Read MoreLead the Conversation, Change the Conversation
Coverage of single-use plastics can be both misleading and demoralizing. Here are 10 tips for changing the perception of the plastics industry at your company and in your community.
Read More
.jpg;width=70;height=70;mode=crop)