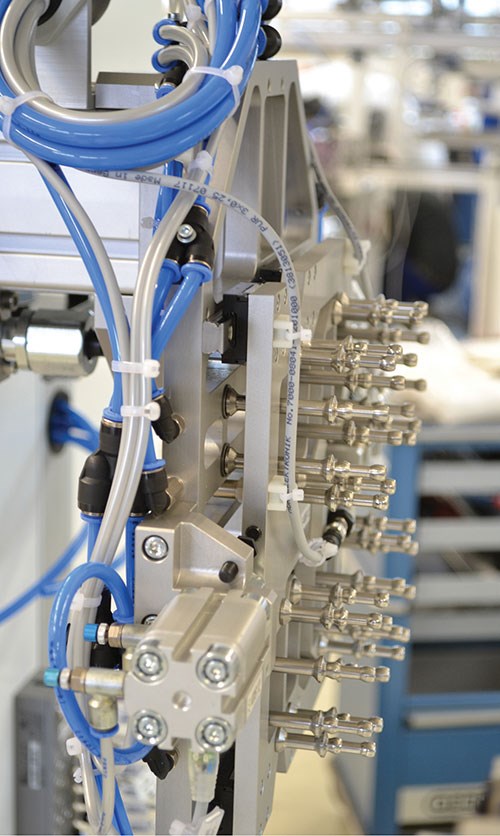
Understanding Automation for Pipette Molding
Feature: Injection Molding
While simple looking enough, the pipette can be challenging to process. They generally have to be produced in high volumes without sacrificing quality due to their demanding applications. Automation is key to making this happen.
Like so many high-volume parts being molded today, the pipette is such a simple and unassuming item. Pipettes are disposable devices used as a fluid-transfer tool in labs and medical environments. They come in a variety of sizes and colors and, while simple looking, they can be challenging to process. The biggest challenge by far, however, is that pipettes generally have to be produced in high volumes without sacrificing quality due to their demanding applications.
In order to create a world-class processing solution, molders first need to understand and face all the challenges before putting anything on paper with their system supplier. To begin with, there are the challenges related to the specifications of the part itself, with regard to dimensions, straightness, and surface defects. Secondly, there are challenges with handling and packaging, which often demand traceability all the way down to the cavity level. There is also a challenge with the operating environment to ensure contamination-free production, which brings with it a battery of GMP (Good Manufacturing Practices) standards. Last but not least is the sheer volume associated with modern production, requiring short cycle times and high cavitation. The sum of all of these factors adds a degree of difficulty that only an efficient and reliable automation system can overcome.
It is important to establish a clear picture of the needs of the system in order to put together an effective and reliable molding cell. If a variety of sizes will be produced on the same system, it is important to include flexibility for fast changeovers. An automation concept always starts with removal of the parts from the mold, but with cycle times below 6 sec and with molds of 32 or even 64 cavities, mold-open time must be kept to an absolute minimum. Beyond that, there are a number of considerations, most of which are expected in today’s marketplace for medical disposables:
•Cavity-specific handling throughout the process.
•Production of pipettes with and without filters.
•Vision inspection of the finished pipettes:
a) Dimensions, flash, run-out, and filter placement;
b) Reject defective parts;
c) Replace with “like cavity” good parts.
•Placement of pipettes in racks:
a) Accommodate a variety of racks;
b) Maintain cavity integrity;
c) Placement of barcode label.
•Palletizing of racks:
a) Placing in stacks or blister packs;
b) Sealing of selling units;
c) Labeling.
THE COST OF QUALITY
A certain expectation for quality has been established in the world of medical disposables. The pipette itself is not so different from years ago, but there are some new terms that are commonplace today and have a very big impact on the production cell.
“Traceability” and “100% inspection” are two such terms that affect the complexity of the cell and the capital investment. These features have moved from the category of “nice to have” to “must have,” and fulfilling these requirements is very expensive.
Consider the breakdown of capital costs for a highly efficient and flexible manufacturing solution. The exact figures will depend on specific requirements, but in general, 100% QC with a vision inspection system, traceability, and GMP requirements can represent around 40% of the investment cost. The painful truth is that these features do nothing to increase output or productivity. They reduce risk, they provide peace of mind, and they allow for verification and validation. The cost justification is rationalized by citing the cost of truckload rejects, product liability issues, and simply “a cost of doing business.” It is nevertheless a “no-value-added” proposition from a productivity standpoint.
By no means should this be interpreted as a suggestion to take shortcuts in quality control or GMP, particularly when molding healthcare products. There are serious implications in substandard products reaching the consumer, and even in the area of diagnostics where the consequences may not be as drastic or direct as for syringes, molders should strive for zero-defect manufacturing practices. Realistically speaking, it is impossible to put a price on protecting the life of a patient or on the image of a company producing pipettes that is an integral part of the overall supply chain. The link between the brand owner and the molder is unbreakable.
THE MOLDING ENVIRONMENT
The operating environment for a pipette solution must ensure a contamination-free process flow. Here, the typical molding areas have progressed from a “white room” to a “clean room,” which again adds expense. Productivity per square foot is a common measurement in manufacturing, but the cost of operating a clean room makes this measurement essential.
The optimum solution will maximize the floorspace utilization while keeping accessibility and serviceability of the system in mind. Naturally, allowing particulates to enter the clean room is unacceptable, but elimination of other types of pollution such as heat and noise is equally desirable.
UNDERSTANDING PRODUCTIVITY AND EFFICIENCY
Use the overall equipment effectiveness (OEE) method of calculating machine efficiency. Since there is a finite amount of time available on a 24/7 basis, your automation equipment supplier will have a limited window of opportunity in which to work. Automation will have no influence over such factors as a “lack of business” or “lack of raw materials” but there are other areas where it can have a profound positive effect on productivity.
The worst enemy of productivity is unplanned downtime of the system. The key to eliminating that is a combination of a robust and reliable system design combined with an excellent preventive-maintenance program. The next positive influence an automation vendor can have is the reduction of planned downtime. It is important to incorporate components that require extremely low or even zero maintenance.
Choose a flexible automation system with a track record of fast changeovers and quick and easy startup. One last consideration is the system’s ability to recover from “micro-stops” of production due to momentary interruptions from, for instance, a lack of filters or racks needed for assembly or packaging. Where possible and practical, the system should automatically restart itself or employ an “adaptive cycle control” to continue production at reduced rates rather than shut down the molding operation.
Opt for a high-speed, precision takeout device that removes the part from the mold with a minimum of encroachment on the mold-open time. Some automation suppliers use lightweight yet stable alloy and composite materials to allow extremely dynamic acceleration and deceleration. In many cases, this dynamic motion allows a certain amount of overlap of the mold and robot movements to safely minimize the mold-open time.
Flexibility begins with the end-of-arm tooling, which can incorporate special technology for efficient cavity-specific sorting. The format parts for the tooling also can have a modular design to allow for fast product changes and easy production restart.
Monitoring and cross-monitoring of the parts being removed eliminates the possibility of mold damage from parts left behind. Individual cavity monitoring and control also allows the user to shut off known defective cavities if necessary without costly downtime.
While many pipette solutions share some common attributes, each system must be custom tailored to the molder’s particular needs for product mix, secondary operations, handling and packaging needs, and labeling. Each solution must take into account the fact that the process is being carried out in a clean room. Therefore, utilization of floorspace is the primary concern. Clean-room compatibility also means paying attention to those details that contribute to contamination so that no particulates are expelled into the atmosphere. It also means fully contained use of compressed air or eliminating air usage altogether.
Equally important is ensuring low noise emissions to enhance the work area. Also look for high-performance drives that incorporate water-cooled servo motors and linear drives to reduce the radiant heat expelled into the clean room. Any heat dumped into the clean room must be removed with expensive air conditioning; water-cooled heat exchangers are much more efficient and operate at a fraction of the cost.
The carrier system for storage, buffering, inspecting, and cavity-specific sorting is one innovative way to optimize space. An ideal carrier should have the capacity to hold a full complement of pipettes for cavity-specific loading of 96 position racks. That means that each carrier would (in the case of a 32-cavity mold) hold 32 x 96 pipette tips, or 3072 tips. As you can see, the density of the tips is very high and is one of the ways to achieve a compact design platform. The carrier concept also allows for transfer to a filter installation station, if necessary, or skips it if not.
This concept also allows for a vision inspection system to check, reject, and even replace pipettes with like-cavity numbers if desired—all before population of the racks, so that only top-quality parts are placed in the rack for further processing. Vision inspection may include top side check, bottom side check, run-out checking, and filter presence, as well as checking for flash or contamination from black specks.
FINISHING THE JOB
Most modern pipette molding automation systems also include the packaging of racks into so-called “selling unit packages.” This may include everything from “bulk pack” to multi-tier racks in a blister pack with customized foil sealing. Look for a supplier than can furnish as many technical solutions to these requirements as you could conceivably require.
When it comes to system supply, service is far more than the repair of a system. It should begin before the first drawing is made. Opt for a supplier that understands the application from start to finish and the quality and manufacturing needs. Chose specialists that understand not only automation, but the medical industry and all of the processes involved—from materials conveying, to injection molding process and mold technology.
Remember that an automation specialist is not a turnkey supplier but rather the integrator of the major components. This makes perfect sense since the automation is the majority of the cell and certainly the most complex part.
It is the attention to detail that allows the automation specialist to identify potential issues along the critical path and take steps to eliminate or at least minimize any delays.
Validation and qualification are essential parts of any medical molding cell. FMEA (Failure Mode & Effects Analysis) and GAMP 5 (Good Automated Manufacturing Practice) science-based risk analysis involve time-intensive, detailed, comprehensive review of the basic areas of design, installation, operation, and performance. Not to be overlooked is the documentation to back up the studies in the language of choice. Here is just a short list of what you can expect:
•Master data with connection diagram and measurements;
•Error messages;
•Manufacturer statement of
conformance;
•Pneumatic plan;
•Safety instructions;
•Electrical interface diagrams and parts lists;
•Control-system hardware overview;
•Input/Output list;
•Functional group listing of the
machine with layout;
•Interface description (Euromap 67);
•Operation of the machine;
•Maintenance and service procedures;
•Setup parameters;
•List of spare and wear parts;
•Sub-supplied component description;
•Operation program.
When discussing a potential project with an automation specialist, insist on a proposal that includes a complete calculation for justification of the capital investment for the application. After all, if it does not make financial sense for you, there is no point in a vendor investing the time to put a “square peg into a round hole.”
Related Content
Adding Remote Service Functions for PET Bottle Blowing
KHS has added features to its internet machine communications portal for PET stretch-blow molding.
Read MoreWhat to Look for in High-Speed Automation for Pipette Production
Automation is a must-have for molders of pipettes. Make sure your supplier provides assurances of throughput and output, manpower utilization, floor space consumption and payback period.
Read MoreAutomation in Thermoforming on the Rise
Equipment suppliers’ latest innovations exemplify this trend driven by factors such as labor shortages, higher-speed thermoformers and tighter quality control.
Read MoreBeyond the Work Center: Using MES for Data-Driven Decisions Across Manufacturing Operations
Move past “is the job running” and “will it finish on time,” and apply MES to inform workflows ranging from accounting, inventory control, planning, material logistics, quality assurance, engineering and shipping.
Read MoreRead Next
Lead the Conversation, Change the Conversation
Coverage of single-use plastics can be both misleading and demoralizing. Here are 10 tips for changing the perception of the plastics industry at your company and in your community.
Read MoreFor PLASTICS' CEO Seaholm, NPE to Shine Light on Sustainability Successes
With advocacy, communication and sustainability as three main pillars, Seaholm leads a trade association to NPE that ‘is more active today than we have ever been.’
Read MoreSee Recyclers Close the Loop on Trade Show Production Scrap at NPE2024
A collaboration between show organizer PLASTICS, recycler CPR and size reduction experts WEIMA and Conair recovered and recycled all production scrap at NPE2024.
Read More