Aptar Seeks FDA Emergency Authorization for N95 Filtering Facepiece Respirator Decontamination with ActivShield
Aptar aims to contribute in the battle against coronavirus with its ActivShield controlled-release chlorine dioxide decontamination strips.
AptarGroup, Inc. (NYSE: ATR), Crystal Lake, Ill., a global leader in drug delivery, consumer product dispensing and active packaging solutions, is seeking U.S. FDA Emergency Use Authorization (EUA) for a solution that allows easy disinfecting of N95 filtering facepiece respirators. As widely reported, the N95 masks are desperately needed by healthcare personnel due to the shortage of disposable masks during the COVID-19 pandemic.
In this simple disinfecting process, the N95 mask and a small strip of Aptar’s ActivShield are placed inside any commonly available one-gallon plastic bag. The strip releases a controlled amount of chlorine dioxide inside the sealed bag to decontaminate the N95 mask. The process takes only three hours until the mask is ready to wear again. It can be performed on-site at the local hospital where the mask is being used.
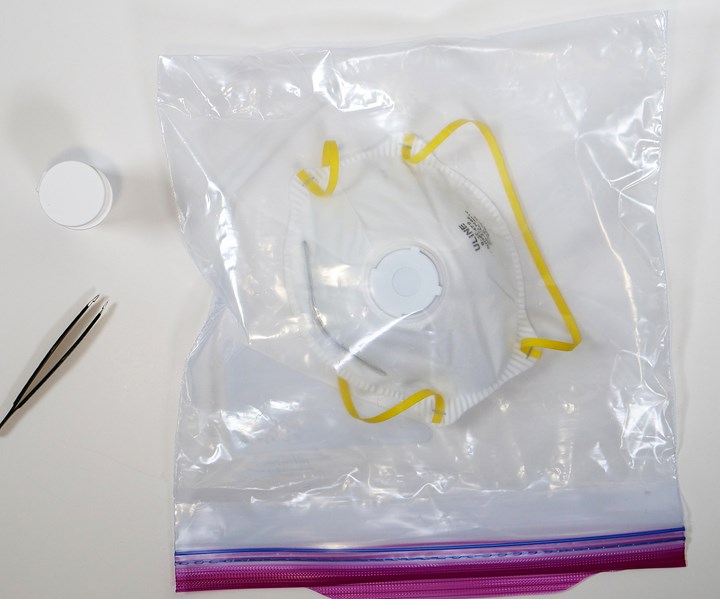
Aptar has submitted its safety and effectiveness data to the FDA for EUA review. The company is working to provide approximately four million ActivShield strips per week and is working to expand its production capacity with the intent to deliver ten million per week by the end of April. “We are extremely encouraged by the promising data generated so far and are eager to deliver this technology to the front line and support the fight against the pandemic,” said John Belfance, President of Aptar CSP Technologies. If the FDA approves the EUA, then ActivShield will immediately become available for this important use.

Related Content
-
Catheter Specialist Finds Sweet Spot Serving Small, Medium-Sized Concerns
Medical-component specialist LightningCath has carved a niche meeting the needs of small to medium-sized entrepreneurs with complex catheter designs … quickly.
-
3D Printed Spine Implants Made From PEEK Now in Production
Medical device manufacturer Curiteva is producing two families of spinal implants using a proprietary process for 3D printing porous polyether ether ketone (PEEK).
-
Compact Solution for Two-Component Molding
Zahoransky’s new internal mold handling technology foregoes the time, space and money required for core-back, rotary table or index plate technologies for 2K molding.












