Cyclic Olefin Copolymer: Alternative Resin For Medical Packaging
With Barex production winding down, processors serving the medical market are eyeing replacements. Some have already commercialized new packages with COC.
Polyacrylonitrile (PAN)-based polymers, best known under the Barex brand, have been a staple of the packaging industry for decades, serving as a barrier and sealant layer in a range of food and medical packaging. However, recent inroads into the food-packaging market by barrier materials such as ethylene vinyl alcohol (EVOH) have eroded PAN’s sales to the point where the sole remaining supplier of the material, Ineos in Lima, Ohio, announced over a year ago that it would stop making the product.
Replacing Barex is an urgent matter for OEMs in the healthcare market due to the regulatory constraints involved in switching packaging materials. A poor choice of packaging material will compromise pharmaceutical purity and potency, so it is essential to identify a material that delivers the required barrier and adsorption profile while also exhibiting low leachables and extractables and meeting regulatory requirements—often in multiple global markets. Significant testing is required when changing pharma packaging materials, hence a high degree of confidence in alternative materials is necessary before undertaking what can be an expensive and time-consuming process with multiple regulatory checkpoints.
Topas cyclic olefin copolymer (COC) from global thermoplastic material supplier Topas Advanced Polymers, Florence, Ky., is one material that has already emerged as a viable alternative to Barex PAN in healthcare and other applications. For several years, Topas Advanced Polymers has worked closely with leading global packaging suppliers to introduce COC polymers in pharmaceutical packaging materials such as medicinal pouches and blisters for patches and other end uses that require strong chemical resistance and low permeation. While COC has already been successfully substituted for Barex in some commercial applications, Ineos’ recent announcement to stop producing Barex has put additional focus and priority on these efforts.
A clear, high-purity thermoplastic with a long, proven track record of successful medical applications, Topas COC is now filling the needs of many healthcare companies who seek a safe and readily available replacement for Barex materials. Although PAN and COC come from the opposite ends of the polymer spectrum, they offer similar barrier/permeation performance and strong chemical resistance. Topas COC is well known for its purity, which is generated via an advanced metallocene polymerization process engineered for exceptionally low leachables and extractables.
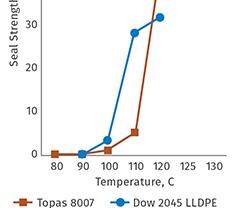
Topas COC delivers broad global regulatory compliance for both healthcare and food-contact applications, and is available in a number of grades to meet specific processing and end-use requirements. The polymer can deliver excellent heat-sealing behavior, facilitating broad use in direct contact with packaged goods. Grades are available with low seal-initiation temperatures for easy sealing, while grades with higher heat resistance are offered for a range of applications that may face significant heat exposure.
KEY PERFORMANCE PROPERTIES
The pharmaceutical packaging industry is already familiar with COC through its use in other flexible, semi-rigid, and container applications such as forming webs, blister packs, and syringes. While not considered a drop-in replacement for PAN, COC delivers effective chemical resistance and low adsorption rates, exhibiting low permeation and migration for medical-grade chemical contents such as nicotine for medicinal patches. COC’s adsorption of nicotine is less than that of glycol-modified polyethylene terephthalate (PETG) and EVOH.
In another example of superlative chemical performance, COC’s resistance to methyl salicylate is similar to PAN and better than PETG. Alcohol resistance is yet another strong suit for Topas COC, with its good alcohol barrier put to the test over the years in many healthcare applications. And the moisture barrier of Topas COC is a known strength, one that has driven its increasing use in healthcare applications such as blister packaging.
Topas Advanced Polymers has developed a web presentation to share its test results . Data on multiple COC grades, as well as competitive materials like PAN, EVOH, and PETG, can be found there. The presentation is updated as new results become available.
Film processors running the COC material have developed innovative nicotine-patch packaging for the transdermal industry. These packaging materials are engineered for excellent product protection that satisfies the needs of the distribution chain and the consumer, while also complying with regulatory requirements. Blister-packaging solutions have also been developed for the rapidly growing e-cigarette market, where potency and nicotine containment are of increasing importance.
Unlike PAN, Topas COC can be easily melt processed on most common thermoplastic processing equipment, including mono and multi-layer film, sheet, and extrusion coating lines, along with injection and blow molding processes. This versatility enables processors to consider Topas COC for a broad spectrum of Barex replacement applications, and allows a range of coextrusion options for film makers who in the past have worked exclusively with Barex laminations.
Further simplifying matters for the film processor is the fact that Topas COC is an ethylene copolymer that adheres well to other ethylene-based polymers such as polyethylene (PE). As a result, tie layers are generally not required in coextrusions. Processing conditions are also very similar to those for PE, making COC resin a good fit for many processors’ existing film extrusion equipment.
HEALTHCARE PACKAGING LEADS THE WAY
Topas COC is a key component in a broad range of global Barex replacement products from leading healthcare packaging companies, including Bemis Healthcare Packaging (BHP), Oshkosh, Wis.; Rollprint Packaging Products, Addison, Ill. (see sidebar, below); and Tekni-Films Div. of Tekni-Plex, Inc., Wayne, Pa.
At BHP, Topas COC is used in the company’s CXB sealant, which was originally created to compete against Barex sealant. When Ineos announced the discontinuation of Barex production, BHP was already prepared to bring solutions to its customers to help ease the transition.
BHP has taken a theoretical, analytical, and practical application approach with many drugs and excipients to best predict a one-to-one packaging solution. CXB sealant is part of a suite of sealants
BHP offers for transdermal, E-Cig, and oral strip applications. Other sealant solutions include sealants based on PE, PET, and Surlyn ionomer resins; however, the CXB sealant, utilizing Topas COC resin, is the strongest Barex alternative in the Bemis portfolio.
Armed with the knowledge of nicotine uptake and resistance, BHP scientists developed an E-Cig cartridge blister package. Bemis SkyBlue CXB blister-forming web and PerfecPharm L-CXB blister lidding both contain CXB sealant. BHP has extensive experience and understanding of the interaction of polymers and nicotine and has been highly successful utilizing CXB sealant for nicotine transdermal and E-Cig cartridge packaging.
The market for Topas Advanced Polymers’ COC materials has expanded strongly since its commercial launch almost two decades ago. Packaging film applications comprise a large share of sales, with commercial examples ranging from twist films for candies, to shrink labels for soft drinks. In the packaging market, the ability of COC to provide complementary properties that ordinary commodity resins cannot deliver, while being relatively compatible with polyethylene, is the key driver behind its utility and commercial success.
Injection-molded healthcare applications for COC comprise another significant part of overall worldwide sales. Prefilled syringes take advantage of the purity and inertness of glass-clear COC, while wearable injectors are an up-and-coming success story for the same reasons. Diagnostic uses for COC are also multiplying, based on its sub-micron detail reproduction capability and low extractable content, as well as high UV and chemical resistance.
Other key uses for COC include optical and electronic applications. Modern mobile devices contain COC in components that are both clear and opaque, ranging from light guide panels to touchscreen films to antennas. Automotive heads-up displays rely on the moisture resistance and clarity of COC.
ABOUT THE AUTHOR: KNEALE
Trained as a chemical engineer, Timothy Kneale has held a variety of product-development and technical leadership roles in the plastics industry. During the last 10 years, he has served as president of Topas Advanced Polymers and overseen sales and market development activities in North America. Contact: (859) 746-6447; info@topas-us.com; www.topas.com.
Related Content
Why Are There No 'Universal' Screws for All Polymers?
There’s a simple answer: Because all plastics are not the same.
Read MoreHow to Select the Right Tooling for Pipe Extrusion
In pipe extrusion, selecting or building a complementary set of tooling often poses challenges due to a range of qualitative factors. Here’s some guidance to help you out.
Read MoreHow to Select the Right Cooling Stack for Sheet
First, remember there is no universal cooling-roll stack. And be sure to take into account the specific heat of the polymer you are processing.
Read MoreWhat to Know About Your Materials When Choosing a Feeder
Feeder performance is crucial to operating extrusion and compounding lines. And consistent, reliable feeding depends in large part on selecting a feeder compatible with the materials and additives you intend to process. Follow these tips to analyze your feeder requirements.
Read MoreRead Next
For PLASTICS' CEO Seaholm, NPE to Shine Light on Sustainability Successes
With advocacy, communication and sustainability as three main pillars, Seaholm leads a trade association to NPE that ‘is more active today than we have ever been.’
Read MoreBeyond Prototypes: 8 Ways the Plastics Industry Is Using 3D Printing
Plastics processors are finding applications for 3D printing around the plant and across the supply chain. Here are 8 examples to look for at NPE2024.
Read More