A Processor's Most Important Job, Part 6: Long-Term Effects
The importance of mold temperature to the development of the desired polymer crystalline structure becomes absolutely crucial in the case of high-performance materials.
This is part six of an eleven-part series focused on the most important job of a processor. To read the rest of this series, click these links:
part one, part two, part three, part four, part five, part seven, part eight, part nine, part ten, part eleven.
As we have indicated in recent articles, the rate at which semi-crystalline polymers develop their crystalline structure tends to correlate with the level of performance that the materials can achieve in the solid state. Materials like PE and PP, often considered to be commodities, crystallize readily due to their low glass-transition temperatures (Tg). The cooling rate of the material can be adjusted to change the degree of crystallinity in the molded part.
However, these changes tend to be relatively small and the longer cycle times that may be needed to manage the slower rate of heat removal may not be justified. As we move up into the semi-crystalline polymers of intermediate performance—aliphatic nylons, acetals and polyesters—the ability of the processor to affect the degree of crystallinity becomes greater, and maintaining the correct mold temperature is more important to part performance.
But it is in the high-performance realm, materials such as polyphthalamides, PPS, and PEEK, where the importance of mold temperature to the development of the desired structure becomes absolutely crucial, and missing the correct mold-temperature setting by even 10-15°F (5-8°C) can result in a part that falls far short of the intended performance of the material. In most cases, the suppliers of these materials provide clear instructions on how the mold temperature must be set to ensure adequate crystallinity. However, occasionally a material supplier will try to let the processor off the hook and in doing so they give some very bad advice.
In our previous article, we discussed the importance of maintaining a mold temperature high enough to ensure a satisfactory level of crystallization in these high-performance materials. Without exception, these mold temperatures cannot be achieved with standard water-circulating units. They require the use of hot oil, electric cartridges, or pressurized water since the required mold temperatures are in the range of 250-400°F (121-205°C), depending upon the polymer and the wall thickness of the part being molded. Many processors consider this a barrier to entry, and material suppliers want to sell resin.
Therefore, sometimes there is a mixed message in the advice coming from the suppliers. The processing community is sometimes told that full crystallization is only important if the part being molded will need to perform at temperatures above the Tg of the polymer. The reasoning goes something like this: An under-crystallized part will never experience performance problems if it is not exposed to a temperature high enough to promote the additional crystallinity that was not achieved due to quenched cooling. According to this advice, the unstable state associated with incomplete crystallization will only come into play if the part becomes hot enough to undergo continued crystallization.
Have questions about materials? To learn more click here.
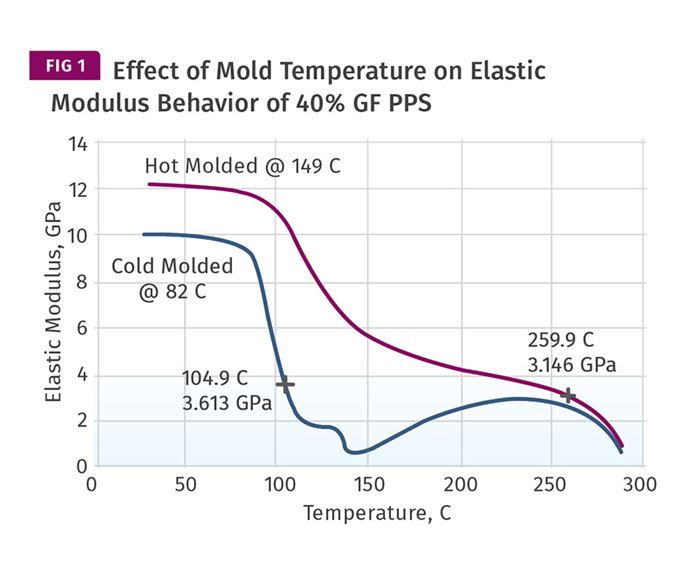
Unfortunately, it is not that simple. Figure 1 shows a comparison of modulus vs. temperature behavior for two specimens produced from a 40-percent glass fiber-reinforced PPS. The mold temperature used for the cold-molded part was 180°F (82°C) while the mold temperature used for the hot-molded part was 300°F (149°C).
The recommended mold temperature in the processing guides for achieving optimal crystallinity is 275°F (135°C). There are some important differences in the temperature-dependent behavior of these two samples that resemble what we see in the PPA material.
The Difference in PPA Material
First, even at room temperature there is an obvious difference in the modulus of the two samples. The part run in the cooler mold has a modulus of 10 GPa (1450 ksi) while the part run in the hotter mold is nearly 25 percent stiffer. But the most important difference occurs as the two samples enter the glass-transition region. The modulus decline begins in both samples at approximately the same temperature. But the way these two samples pass through this transition is very different.
The sample molded in the hotter mold exhibits a modulus decline of a little over 50 percent by the time the temperature reaches 302°F (150°C). It then establishes a new plateau that extends to 500°F (260°C) before displaying a second decline associated with the initial stages of crystal melting. The part produced in the cooler mold exhibits a much sharper decline in modulus that ultimately results in more than a 90-percent reduction in the room-temperature stiffness before the material reaches 302°F. The upward trend in the modulus beyond this point represents the attempt of the polymer to form the crystals that should have been created during the molding process.
The logic behind the advice that optimal crystallinity does not matter if the application temperature never reaches the Tg may appear to make sense since the modulus of the two samples changes in a similar fashion up to a temperature of about 185°F (85°C). However, it is important to understand that the behavior of a material over time when under constant or cyclic loading will reflect the way the material responds to an increase in temperature.
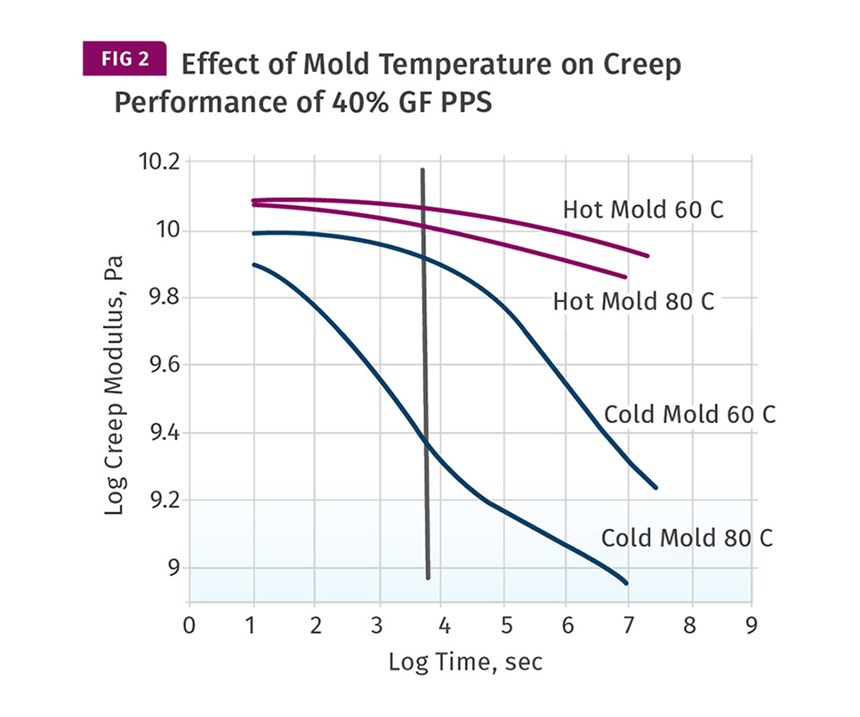
Consequently, the earlier and sharper decline of the modulus in the sample produced in the cooler mold will be reflected in creep tests performed at the low temperatures leading up to the glass transition. This can be seen in Figure 2, which shows plots of apparent modulus, sometime referred to as creep modulus, as a function of time for samples produced at both mold temperatures when tested at temperatures of 140°F (60°C) and 176°F (80°C). The differences are striking and very important to the long-term integrity of the molded part. The samples run in the hotter mold show a negligible change in apparent modulus over the tested times of 4-12 months. The samples run in the cooler mold undergo very rapid changes that result in nearly an order of magnitude decline in apparent modulus over this time frame. The vertical line drawn through the creep curves represents the 2-hour point. Even at this early stage of the tests, the differences in performance as a function of the mold temperature at which the samples were produced is evident, particularly for the tests conducted at 176°F.
These results show that any suggestion that optimal crystallinity does not matter at lower temperatures is simply misguided thinking that arises largely from the failure to take into account the full spectrum of performance as a function of temperature, time and applied load.
We have spent a lot of time discussing crystallinity and there is a lot more that we could say. While the cooling rate controlled by the temperature of the mold is the most important variable, crystallization can be influenced by the stresses associated with pack and hold pressures, the effects of orientation and a process known as nucleation. Crystallization is also a process that releases heat, lengthening the cycle time in unexpected ways. Next, we will close out the conversation on crystallinity with a brief review of these other factors.
About the Author
Michael Sepe
Michael Sepe is an independent, global materials and processing consultant whose company, Michael P. Sepe LLC, is based in Sedona, Arizona. He has more than 40 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting and failure analysis. Contact: 928-203-0408 • mike@thematerialanalyst.com.
Related Content
Back to Basics on Mold Venting (Part 2: Shape, Dimensions, Details)
Here’s how to get the most out of your stationary mold vents.
Read MoreThe Importance of Barrel Heat and Melt Temperature
Barrel temperature may impact melting in the case of very small extruders running very slowly. Otherwise, melting is mainly the result of shear heating of the polymer.
Read MoreA Systematic Approach to Process Development
The path to a no-baby-sitting injection molding process is paved with data and can be found by following certain steps.
Read MoreWhere and How to Vent Injection Molds: Part 3
Questioning several “rules of thumb” about venting injection molds.
Read MoreRead Next
A Processor's Most Important Job, Part 5: POM Polymers
Using a mold temperature above a polymer’s Tg ensures a degree of crystallinity high enough to provide for dimensional stability, even if the part must be used at elevated temperatures. But POM is an exception. Why?
Read MoreA Processor's Most Important Job, Part 7: Reviewing Crystallinity
There are several process-related issues that influence crystallinity besides cooling rate. Let’s examine a few.
Read MoreMaking the Circular Economy a Reality
Driven by brand owner demands and new worldwide legislation, the entire supply chain is working toward the shift to circularity, with some evidence the circular economy has already begun.
Read More
.jpg;width=70;height=70;mode=crop)