Research Reaffirms FDA Rules for Virgin PET Cap as Barrier
Study refutes previous research that raised doubts over V-PET’s effectiveness as a functional barrier layer when coextruded with R-PET.
A 2016 European study raised doubts about the effective- ness of virgin PET as a barrier cap layer in a multi-layer package also containing post-consumer reclaim (PCR). But more recent research using computer simulations has reaffirmed guidelines issued in 2006 by the U.S. Food and Drug Administration (FDA). Those guidelines, Guidance for Industry: Use of Recycled Plastics in Food Packaging: Chemistry Considerations state that with the right structural elements, a virgin skin cap layer indeed performs as an acceptable functional barrier over PCR materials for food contact.
The results of this more recent study were presented first by PTi/Processing Technologies International (PTi), Aurora, Ill., in December at Plastics Technology’s Extrusion 2016 conference. PTi conducted the study last year in collaboration with Plastic Technologies, Inc. (PTI), Holland, Ohio, and Container Science Inc., Atlanta. Plastic Technologies Inc. is recognized worldwide as a leading source for preform and package design, package development, rapid prototyping, pre-production prototyping, and material evaluation engineering for plastics packaging. Container Science Inc. was founded in January 2002 to offer materials-science expertise for improving the basic performance, quality, and economics of PET and other plastic container materials. The company (containerscience.com) provides a fundamental knowledge of the chemistry and science associated with plastic containers, and translates that understanding into practical solutions that address the needs, issues, and opportunities for this industry.
This study was prompted by a recent 2016 European industry report by Dr. Frank Welle of the Fraunhofer Institute. Titled Assessment of Recyclates Behind Functional Barriers and presented last March at the PET Recycling for Food Contact Conference, the report questioned the efficacy of an A-B-A structure with PET recyclates behind a functional barrier and suggested that the virgin cap layer may become contaminated during extrusion, rendering the functional barrier inadequate. The European report findings were based on testing of recycled PET packaging at elevated use temperatures as high as 212 F (100 C), which is not in keeping with guidance stipulated by a 2006 FDA recommendation (i.e.; room temperature and below) using a minimum of 1-mil-thick virgin PET cap layer to encapsulate the PCR PET materials for direct food-contact packaging applications.
The PTi study set out to examine the elevated application temperature relevance used as part of the Fraunhofer Assessment and reaffirm virgin PET for suitability as a functional barrier and its corresponding FDA guidelines. The simulation results demonstrate that a 1-mil virgin cap layer is adequate protection for a PET food package when used at room temperatures. In coextruded multi-layer PET structures, the FDA recommended in 2006 a 1-mil-thick virgin cap layer for room-temperature applications, and a 2-mil-thick cap layer for higher-temperature use (up to 302 F/150 C) to prevent permeation of contaminants migrating from the PCR PET regrind core layer of the packaging material into the contained food. The virgin cap layer provides protection from unhealthy contact or transfer of inks, adhesives, chemicals, or other materials not meant for consumption.
The simulation was focused on migration in the die and feedblock. It was done at the typical melt temperature of PET: 525-550 F. The Fraunhofer report mentioned migration measurements that were done at 212 F and came to conclusions about migration during the extrusion process. In reality amorphous PET packages are used at room temperature and it would be relevant to measure migration or perform challenge tests at room temperature. At the elevated temperature of 212 F, the amorphous PET package would lose structural integrity.
THE METHODOLOGY
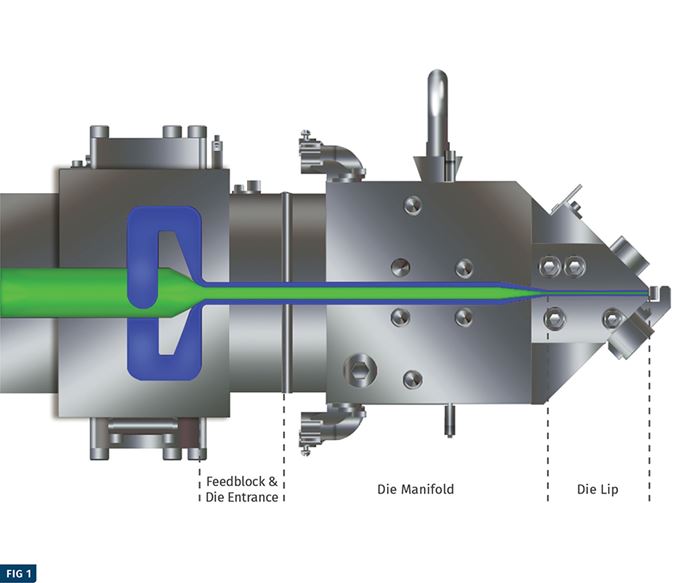
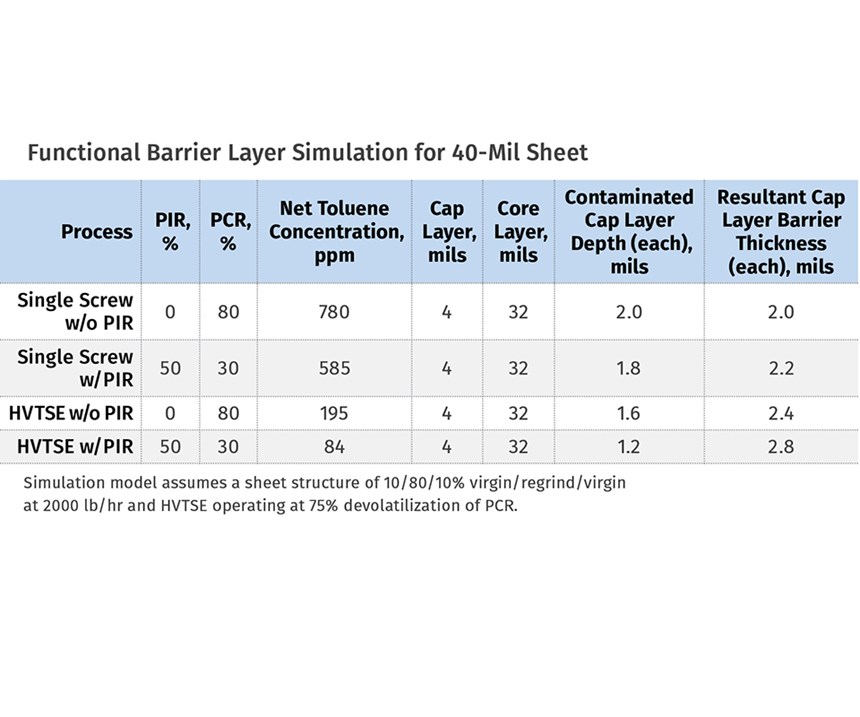
PTi performed four simulations of the FDA recommended challenge test (coextrusion of virgin/PCR/virgin with 780 ppm of toluene in PCR) as part of this study. The accompanying illustration depicts a model of the feedblock and die that was used to simulate expected permeation of toluene out of the core PCR layer. The graphs on pg. 54-55 show the predicted concentration of toluene in the core and cap layers at the end of the extrusion process for the four simulations. The accompanying table summarizes data from four different simulations that modeled the production of a 40-mil-thick PET sheet with a 10/80/10% virgin/ regrind/virgin layer structure at a combined rate of 2000 lb/hr.
The first simulation assumed a conventional single-screw extruder (i.e.; no devolatilization capability) processing the 80% core layer using only PCR PET flake. The second simulation changed the formulation to a 50% post- industrial reclaim (PIR) and 30% PCR PET flake blend. The third simulation repeated the formulation used during the first simulation, while adding the devolatilization benefits of the high-vacuum twin-screw extruder using a patented Bandera HVTSE co-rotating twin screw. And finally, the fourth simulation repeated the formulation of the second simulation while also adding the benefits of the HVTSE process.
For each of these four simulations, the results demonstrate that the virgin cap layers maintain their suitability as a functional barrier, since the resultant uncontaminated virgin cap-layer thicknesses exceed the 1-mil FDA recommendation. The third and fourth simulations further demonstrate the devolatilization benefits using the HVTSE dryer-less process, which is shown to increase the resultant uncontaminated virgin cap-layer thicknesses (i.e.; the resultant functional barrier-layer thickness) due to a significant reduction of contaminant concentration levels in the core layer.
In summary, this study effectively demonstrated the importance of various factors with regard to contaminant migration, the most significant being the correlation of higher migration rates at increased application temperatures. In the Fraunhofer Assessment, it was reported that the permeation rate at 212F (100 C) was rapid (contaminant breakthrough time through a 0.5-mil cap layer was about 1 day), but at room temperature the rate was much slower (equivalent contaminant breakthrough time was predicted to be greater than 100 years).
Other conclusions from this study indicate that exposure time during extrusion processing has some impact, with the simulation models showing primary contaminant migration occurring in the feedblock and die area. However, in this area migration is found primarily at the interface and not throughout the virgin cap layer.
Furthermore, this study demonstrates that virgin cap layers can remain uncontaminated at a layer thickness greater than 1 mil, proving the functional barrier remains intact during the extrusion process and reaffirming FDA guidelines for use as an effective barrier. Simply, with the right structural elements, a virgin skin cap layer performs as an acceptable functional barrier over PCR materials for food contact in accordance with the original guidelines established by the FDA.
Ultimately, food packagers/sheet processors should demonstrate to the FDA that their manufacturing process and package produced meet the relevant food safety requirements.
ABOUT THE AUTHOR: Sushant Jain is senior scientist, applications and technology, for Processing Technologies LLC, Aurora, Ill., a leading supplier of complete sheet extrusion systems. Jain has 30 years of extensive experience in the plastics industry. He has held leadership roles in R&D, product and process development, and lean manufacturing with leading packaging companies such as Pactiv, American National Can, Amoco Foam Products, and Continental Can. He has developed and commercialized containers for food/ nutritional products. Contact: (630) 585-5800; sjain@ptiextruders.com; ptiextruders.com.
Related Content
Extrusion Excellence: This Year's Top Stories
Revisit the year’s most popular articles on extrusion technology and processes, showcasing innovations, best practices, and the trends that captured the plastics processing community’s attention.
Read MoreBrewer Chooses Quick-Change Flexibility to Blow Wide Range of PET Beer Bottles
Beermaster Brewery found a “universal” stretch-blow machine from PET Technologies enables multiple changes per day among four sizes of beer bottles.
Read MoreHow to Decrease the Extrudate Temperature in Single-Screw Extruders
In many cases, decreasing the discharge temperature will improve product quality and perhaps even boost rate. Here are ways to do it.
Read MoreHow to Effectively Reduce Costs with Smart Auxiliaries Technology
As drying, blending and conveying technologies grow more sophisticated, they offer processors great opportunities to reduce cost through better energy efficiency, smaller equipment footprints, reduced scrap and quicker changeovers. Increased throughput and better utilization of primary processing equipment and manpower are the results.
Read MoreRead Next
See Recyclers Close the Loop on Trade Show Production Scrap at NPE2024
A collaboration between show organizer PLASTICS, recycler CPR and size reduction experts WEIMA and Conair recovered and recycled all production scrap at NPE2024.
Read MoreFor PLASTICS' CEO Seaholm, NPE to Shine Light on Sustainability Successes
With advocacy, communication and sustainability as three main pillars, Seaholm leads a trade association to NPE that ‘is more active today than we have ever been.’
Read More