Polymer Science for Those Who Work With Plastic — Part 1: The Repeat Unit
What are the basic building blocks of plastics and how do they affect the processing of that material and its potential applications in the real world? Meet the repeat unit.
When we drive a vehicle, we often don’t think much about how the vehicle works. We get in, turn it on and start driving. Our main concern is getting to our destination. That approach works until something goes wrong. If you have ever had this conversation with your mechanic, “Umm, it’s making a clunking noise up front by the tire,” and he looks at you funny, then you know exactly what I mean. The problem is that you are clueless on what needs to be fixed and how to fix it.
The bad news is that many of us who work with plastics approach the materials in the same way we do our vehicles: We don’t want to know much about how the plastic works, we just want it to work. That “how it works” is often regarded as the “black box” of plastic materials, leading some people to conclude that processing plastic materials is an art rather than a science. Frequently this functional approach gets the job done, but sometimes we hit that spot in our professional lives where we can’t figure something out. It could be any number of things: lot-to-lot variations; unexplained warpage; increasing cycle times; parts failing unexpectedly — the list goes on ad infinitum to the point you think there is a poltergeist on the shop floor.
The good news is that with a little bit of time (and I do mean a little bit) spent learning some key polymer science ideas, you can be significantly more prepared to tackle those unexpected and seemingly unexplainable problems you encounter when working with plastic materials.
Introduction to Polymers: The Repeat Unit
All plastic materials are comprised of polymer molecules, and polymer science is the study of those polymer molecules. Typically, when we are talking about the material or application side of things, we call it a plastic, but if we call that same plastic a polymer then we are referring to the molecules that make up that plastic. Everything is made up of something. Consider a snowball. It consists of thousands of snowflakes that are pressed together into a ball. Or you could also consider a loaf of bread, which is made primarily of flour, water and a little bit of leaven. If you wanted to know more about the snowball — or the loaf of bread — you should investigate the key ingredients from which they are made. Because all plastics are made of polymers, you will benefit from knowing the basics of how polymers work.
By now, you might be asking, what are polymers? Polymers are very, very long molecules made up of identical small parts that repeat over and over again, hundreds or thousands of times, usually in a linear fashion, like a necklace. Those identical small parts that repeat over and over again have a special name: the repeat unit.
Each repeat unit is made of a unique combination of atoms. A real-world comparison would be a pearl necklace. The pearls are identical and repeat over and over again to make one long, thin necklace. One pearl is a small portion of the much larger system. On the molecular level, one polymer repeat unit is like a very tiny pearl. Interestingly, just like you can change necklace types (that is, chain link, beaded and more), you can change the type of repeat unit for a polymer. When you change the type of repeat unit, you change the type of plastic.

Figure 1 – Two carbons and four hydrogens are all that constitute the PE repeat unit. Source (all images): David Rhoades
The simplest of all polymer repeat units is the ubiquitous polyethylene (PE), which has a broad array of applications, including films, pipes, bags, drink containers, garbage bins, toys, electronics and so on. The PE repeat unit is so simple because it is made of two carbons and four hydrogens (Figure 1). You may even argue that this repeat unit could be further reduced to one carbon and two hydrogens, but that would be unconventional. The way it’s drawn — with two carbons and four hydrogens — is reminiscent of the monomer this polymer is synthesized from (more on that in a future article).

Figure 2 – In the PVC repeat unit, the only difference with PE is the substitution of one hydrogen with a chlorine.
Another simple polymer repeat unit to consider is polyvinyl chloride (PVC). This polymer also has many applications, but some of the most familiar ones are shower curtains or pipes. The most significant difference between PE and PVC is that one of the hydrogen atoms is replaced with one chloride atom (Figure 2). This small difference has a profound effect on the properties of the plastic material.
Many differences are created when switching from a hydrogen in PE to a chlorine in PVC. I will highlight only two.
First, the chlorine atom is very different than a hydrogen atom in that it has a strong electronegative force, which results in strong intermolecular forces (IMF). IMFs are like tiny magnets all up and down the polymer molecule that draw them together and keep them stuck to each other. The net effect of this is that it takes significantly more thermal energy to make the molecules moveable and processable.

Table 1 – Approximate values adapted from Polymer Physics by Ulf Geddes, referencing the Schmieder and Wolf study.
In technical terms, this effect is altering the glass transition temperature, annotated as Tg. Back in the 1950s, a research group undertook an interesting study where they systematically increased the chlorine content of PE. Can you imagine what happened? Increasing the percentage of chlorine caused a linear increase in the glass transition temperature or Tg (see Table 1) (reference: Schmieder and Wolf). This higher Tg for PVC is the reason why plasticizers are often added to the material.
Plasticizers are small molecules that disrupt the IMF, preventing those tiny magnet-like things from finding each other. Increasing the amount of plasticizer in the PVC has the opposite effect of adding chlorine content, and it serves to decrease the glass transition of the polymer, which alters its potential application — consider again the shower curtain versus the pipe.
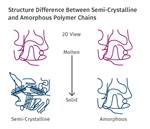
A second difference between PE and PVC that stems from the repeat unit is that of crystallinity. PE is known for being highly crystalline. Some polymers can be quench cooled to keep them from crystallizing, like PETE, but that’s difficult to do with PE. What about the crystalline content of PVC? PVC does not crystallize but is amorphous. What is the reason for that, you might ask? All we did was switch from a hydrogen to a chlorine. It turns out that the switch of atoms causes the repeat unit to be arranged in a haphazard manner such that the polymer molecule is too disordered to crystallize. Without the ability to crystallize, the PVC plastic has increased dimensional control. Dimensional variations such as shrink and warp are often attributed to crystallization.
In summary, understanding the polymer repeat unit of your plastic material is a solid first step in demystifying the ambiguities that can occur with plastic materials. You can easily determine the repeat unit of your plastic material by doing a web search of the name of the plastic in question and the word “microstructure.” Usually, an image of the repeat unit will appear. When you do that, look for features that differentiate it from the PE and PVC molecules. Subsequent articles will build upon the ideas started here. Next time, we will look at the length of the polymer molecule and how that affects its performance.
Related Content
Improving Twin-Screw Compounding of Reinforced Polyolefins
Compounders face a number of processing challenges when incorporating a high loading of low-bulk-density mineral filler into polyolefins. Here are some possible solutions.
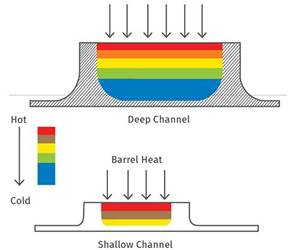
Read MoreThe Importance of Barrel Heat and Melt Temperature
Barrel temperature may impact melting in the case of very small extruders running very slowly. Otherwise, melting is mainly the result of shear heating of the polymer.
Read MoreBack to Basics on Mold Venting (Part 1)
Here’s what you need to know to improve the quality of your parts and to protect your molds.
Read MoreUnderstanding Melting in Single-Screw Extruders
You can better visualize the melting process by “flipping” the observation point so the barrel appears to be turning clockwise around a stationary screw.
Read MoreRead Next
A Quiet Revolution In Materials Characterization
In December, about a dozen representatives of European chemical companies gathered in Aachen, Germany, to celebrate the 20th anniversary of a quiet revolution in plastics.
Read MoreInjection Molding: Melting Amorphous vs. Semi-Crystalline Plastics
Understanding the differences in how each melts is crucial to obtaining melt uniformity.
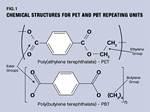
Read MorePBT and PET Polyester: The Difference Crystallinity Makes
To properly understand the differences in performance between PET and PBT we need to compare apples to apples—the semi-crystalline forms of each polymer.
Read More