Tracing the History of Polymeric Materials: Acrylic
How acrylic was born.

Acrylic polymers were developed in Germany over the course of around 70 years, despite technical and economic hurdles and battles over intellectual property and professional pride. (Photo: Evonik)
During the era when many of the polymers we have discussed previously (nylon, PET, PBT, PC, acetal) were undergoing development, another member of the family that has become a mainstay in the industry was following its own lengthy path to commercialization. This was the material that has become known generically as acrylic.
The process from the first synthesis of acrylic acid, the base from which the monomer is derived, to introduction of the commercial polymer, was an 85-year journey. Even after commercialization of the first compounds, the process of getting to the acrylic materials that we are familiar with today was fraught with a number of technical and economic hurdles, as well as battles involving intellectual property and professional pride. The process was driven almost exclusively by German chemists; even when contributions were made by scientists from other countries like England, they were working in or had been educated in Germany.
Acrylic acid was first synthesized in the laboratory in 1843. Methacrylic acid was derived from acrylic acid in 1865 and shortly thereafter it was reacted with methanol to produce the compound known as methyl methacrylate. This is the monomer that is the primary ingredient in what would eventually become the acrylic polymer. The accompanying figure shows the chemical structure of these three compounds. Both methacrylic acid and methyl methacrylate contain the double bond that is crucial in promoting polymerization. In 1877, the German chemist Wilhelm Rudolph Fittig, Professor of Chemistry at Tübingen University, discovered the process for polymerizing methacrylic acid.
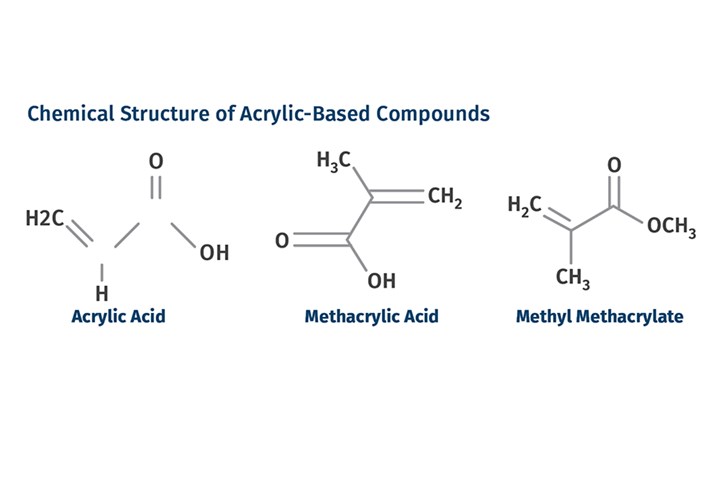 Acrylic acid was first synthesized in 1843, methacrylic acid in 1865, and methyl methacrylate monomer soon after. Acrylic polymers as we know them didn’t appear until the 1930s. (Image: M. Sepe)
Acrylic acid was first synthesized in 1843, methacrylic acid in 1865, and methyl methacrylate monomer soon after. Acrylic polymers as we know them didn’t appear until the 1930s. (Image: M. Sepe)The commercial significance of the material was not immediately recognized. At the time there was not a clear understanding of the composition of materials in this chemical family, and in particular there was poor comprehension of the difference between the organic acids and their esters. Today we know that esters of organic acids are more difficult to polymerize but produce more stable products. But the distinction between acrylic acids and their esters was not well understood at the time.
In 1892, Georg Kahlbaum, a professor at Basel University, carried out research on methyl acrylate, which he polymerized into polymethyl acrylate, the precursor to our PMMA of today. The initial polymers were not of very high molecular weight, taking the form of high-viscosity, gelatinous materials whose utility was not apparent at first. But it was noted that as the polymer formed, the clear monomers remained clear in polymerized form.
One of the big challenges was developing an efficient method for manufacturing the acrylic acid esters to be used as monomers.
In 1901 Otto Röhm published his doctoral dissertation on polymerization products of acrylic acid. Röhm’s thesis advisor at the beginning of his doctoral studies was Hans von Pechmann, also a Professor of Chemistry at Tübingen University and the person who had first created polyethylene in 1898 while studying diazomethane. Von Pechmann was persuaded that acrylic-based materials had the same potential as other notable successes of the time such as rubber, celluloid, and casein. He asked Röhm to conduct his doctorate research on esters of acrylic acid. Röhm quickly figured out that methyl acrylate polymerized more easily than ethyl acrylate and focused on creating a solid material using a combination of light, heat, and a chemical initiator. He produced a colorless, transparent material that had characteristics “between tough, flexible glass and stiff rubber.”
If von Pechmann had remained as Röhm’s advisor, Röhm would likely have transitioned smoothly into an academic position at Tübingen. But by the fall of 1901, when Röhm was concluding his work, von Pechmann left the university, having been diagnosed with “severe melancholy,” what today would be called depression, and took his own life in April of 1902. Röhm set aside his doctorate, but appeared to have an instinctive appreciation of the potential for these materials, and in 1907 he teamed up with banker Otto Haas to form the Rohm & Haas chemical company, a name which became synonymous with acrylic polymers.
The initial focus for the use of these materials was in textiles and leather goods. Röhm used the proceeds from the sales of these products to resume his acrylic chemistry research. But the history of the development of modern acrylic polymers was characterized by Röhm’s approach as a businessman rather than a chemist. This led to long periods of attempts to develop products that reflected a poor understanding of the acrylic chemistry because the focus was on developing a product that was marketable. Röhm observed that the acrylic polymers he was creating had a rubber-like set of properties.
Aware of the success of natural and synthetic rubber, Rohm sought to produce an ester-based rubber using the mechanism of vulcanization. In 1912 Röhm received a German patent for the “process for manufacturing a product with the properties of vulcanized rubber.” A special rolling mill was installed at Rohm & Haas to carry out the vulcanization process. But the patent was not technically viable because vulcanization relies on unsaturated sites where the sulfur can attach chemically to create the crosslinked structure. Röhm did not realize that the ester-based polymers were saturated structures that could not be vulcanized.
Rohm observed that the acrylic polymers he was creating had a rubber-like set of properties.
Nevertheless, in 1915, Röhm secured a German patent for the use of polymerized acrylic acid esters as paint binders and for use in drying oils in industrial paints and lacquers. During this time period, Röhm came up with about 20 different proposed routes for creating useful products based on acrylic acid chemistry. By 1918 he had brought on six chemists to work through these ideas. One of these chemists was Dr. Walter Bauer, who became Röhm’s point man for all things acrylic. Bauer dutifully worked through Röhm’s proposals, but had a much better fundamental understanding of organic chemistry. After going through Röhm’s laundry list without success, he began to work on his own ideas for synthesizing acrylic esters.
One of the big challenges was developing an efficient method for manufacturing the acrylic acid esters to be used as monomers. Many experimenters had started with organic acids, aldehydes or ketones that had chemical structures similar to acrylic acid. These various approaches were plagued by poor yields. Bauer developed a complex multi-step process dubbed the ethylene approach. Because ethylene was difficult to obtain at the time, Bauer started with acetylene, reacting it with hydrogen bromide to form ethylene bromide. This was a route that most chemists felt was fruitless, but Bauer showed that in the presence of the appropriate catalysts, it worked.
This reaction, which was only the beginning of the process, became the subject of two patents issued to Rohm & Haas in 1919 and 1921. The process concluded with an innovative approach to the chemistry that was called the “one-pot process.” In doing so, Bauer exploited a chemistry that had been developed in 1913 but had problems with yield that he was able to solve, making the route to commercial application viable.
Bauer, in commenting on his experiences in investigating new approaches to chemistries that were thought to be useless, delivered a quote that should resonate with all innovators: “Doubts about the accuracy of existing thinking prove to be justified again and again. Research scientists frequently need to act in a way that appears irrational.”
The monomer that Bauer succeeded in synthesizing was ethyl acrylate. While the breakthrough was made in the very early 1920s, a combination of technical challenges related to polymerization, economic difficulties in Germany during the 1920s, and a healthy dose of paranoia regarding the intellectual property associated with the one-pot process, delayed the development of useful commercial products for about a decade. And that part of the story will be told in our next installment.
ABOUT THE AUTHOR: Michael Sepe is an independent materials and processing consultant based in Sedona, Ariz., with clients throughout North America, Europe, and Asia. He has more than 45 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting, and failure analysis. Contact: (928) 203-0408 • mike@thematerialanalyst.com
Related Content
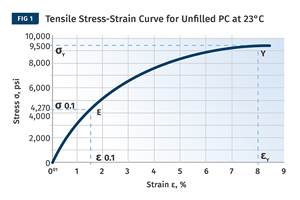
The Effects of Stress on Polymers
Previously we have discussed the effects of temperature and time on the long-term behavior of polymers. Now let's take a look at stress.
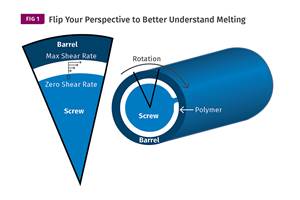
Read MoreUnderstanding Melting in Single-Screw Extruders
You can better visualize the melting process by “flipping” the observation point so the barrel appears to be turning clockwise around a stationary screw.
Read MoreA Systematic Approach to Process Development
The path to a no-baby-sitting injection molding process is paved with data and can be found by following certain steps.
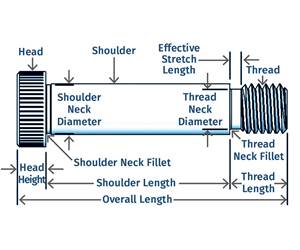
Read MoreWhy Shoulder Bolts Are Too Important to Ignore (Part 1)
These humble but essential fasteners used in injection molds are known by various names and used for a number of purposes.
Read MoreRead Next
Tracing the History of Polymeric Materials: The Differences Between Nylons & Polyesters
In many respects, nylons and polyesters appear to be interchangeable. But there are interesting differences in the properties of these two families that arise from their chemical structures.
Read MoreTracing the History of Polymeric Materials: Aliphatic Polyketone
Aliphatic polyketone is a material that gets little attention but is similar in chemistry to nylons, polyesters and acetals.
Read MoreTracing the History of Polymeric Materials: Acetal
The road from discovery in the lab to commercial viability can be long, and this was certainly the case for acetal polymers.
Read More
.jpg;width=70;height=70;mode=crop)