Tracing the History of Polymeric Materials: More on Phenolic
Creation of an invention like phenolic can be traced through a long chain of events and contributors. Here’s the full story.
A review of the history of technological developments shows that breakthroughs do not happen in a vacuum. Different contributors take small steps, which are then advanced by others, and when an invention like phenolic comes together in its final form, its creation can be traced through a long chain of events. It is also true that multiple people often have the same idea, and the person that history remembers is often the one that first receives formal recognition for putting the last piece of the puzzle in place, rendering the development both technologically feasible and economically viable.
Before Leo Baekeland in the U.S. had even started on his investigation into what would become phenolic chemistry, Arthur Smith obtained a British patent in 1899, the first one to be issued in the endeavor to produce a useful phenolic. However, it required several days to harden at a temperature of 90-100 C and distorted in the process. At the same time that Baekeland was working on refining the reaction between phenol and formaldehyde, a German chemist, Carl Heinrich Meyer, produced an acid-catalyzed reaction between phenol and formaldehyde, but its use was limited to lacquer and adhesives.
An Austrian chemist named Adolf Luft had been working on the same problem. But the compound that Luft came up with used camphor as the solvent and was very brittle. A British electrical engineer, James Swinburne, worked for three years to find a solvent that would correct this shortcoming and finally came up with caustic soda as the solution. He arrived at the British patent office just a little too late to become the historical figure remembered for the creation of phenolic. In fact, Baekeland preceded him by one day.
Although rivals and potential adversaries, Baekeland and Swinburne ended up working together after Baekeland initially threatened patent litigation when Swinburne set up a plant in the U.S. In fact, Baekeland managed to maintain a dominant position in the market through a combination of patent litigation threats, the granting of permission for the use of his patents to Swinburne and others during World War I, and ultimately buying up many of his competitors in the late 1920s just as his patents were about to expire.
The route that Baekeland and Swinburne took to the process of manufacturing phenolics was a reflection of the difficulty in controlling a condensation polymerization reaction. Condensation polymerizations typically produce unwanted by-products that can hinder the desired reaction and must be removed or suppressed. The problem of managing this aspect of the chemical reaction was dramatically illustrated by the experience of the German chemist Adolf von Baeyer. Baeyer is primarily remembered for his synthesis of indigo, and he won the Nobel Prize in chemistry in 1905. He also was also a protégé of August Kekulé, the famous chemist mentioned in last month’s column, whose assistant mentored Baekeland through his doctorate. Baeyer is credited with being the first person to investigate the chemical reaction between phenol and formaldehyde in 1872. The violent chemical reaction produced a resinous tarlike solid that Baeyer discarded after he was unable to analyze its composition.
The moldability of phenolic gave birth to the discipline of plastic design.
That could have been the end of the road for formaldehyde-based polymers, had it not been for another accidental discovery made by Bavarian chemist Adolf Spitteler 25 years later. A cat that resided in Spitteler’s lab knocked over a bottle containing an aqueous solution of formaldehyde, spilling the contents into a saucer of milk. Spitteler observed that the milk quickly curdled into a hard compound that appeared to have properties similar to those of celluloid. The chemical reaction that produced this material involved the crosslinking of a mixture of proteins known as casein by the formaldehyde and the polymer became known as casein. The discovery that formaldehyde rendered casein insoluble in water had actually been made four years earlier in 1893 by a French chemist, Alfred Trillat. But the historical credit goes to Spitteler and a non-chemist collaborator, Wilhelm Krische.
Krische was searching for a material that he could use to make washable white writing boards. He had already tried using casein, and while it worked initially, the casein softened the first time the whiteboard was wiped clean with water. The crosslinked material solved this problem and the market was so significant that Spitteler and Krische founded a company to make casein and related products. Trillat had tried to convince a French company to manufacture the product that had come from his research, but he was not able to generate the needed interest. The German company’s success, coupled with the realization that casein could easily be fabricated into a wide variety of shapes, prompted the belated founding of a competing French operation.
The commercial product was named Galalith (“milk stone” in Greek). The material was displayed at the Paris Universal Exhibition in 1900 and was patented in 1906. There is no historical information indicating that the German and French companies litigated for the rights. They both produced the material to meet a growing market, primarily in the fashion industry to make buttons, buckles and jewelry, although casein found its way into a lot of products that also used celluloid, such as combs and knife handles. It was even used to make electrical insulators before the advent of phenolic.
For all of its success, and the fact that it preceded phenolic by over a decade, casein was still a material in the same vein as rubber and celluloid, a modification of a naturally occurring material and not a true synthetic product. However, it was much easier to produce than phenolic because the proteins, consisting of alpha, beta, and kappa-casein, are already polymers with molecular weights in the range of 20,000 to 25,000 g/mole. Phenol has a molecular weight of only 94, requiring the formation of a prepolymer prior to crosslinking.
Polycarbonate was not the first product made by GE Plastics. It was phenolic.
As a side note, those who have been in the plastics industry for more than 15 years remember a time when General Electric had a plastic materials division. When asked about the history of GE Plastics, even most of us old timers will point to the advent of polycarbonate in the mid-1950s. The story of that development was told in a commercial that ran a lot on Sunday morning news shows in the 1990s and showed a cat walking through a lab in the middle of the night. The cat knocks over a bottle and in the morning a scientist, presumably Dan Fox, comes into the lab to find a clear glob of material that he then subjects to boiling water, flame, and a hammer, all of which fail to affect the integrity of the material.
While it is true that polycarbonate was one of those accidental discoveries, there was no cat. The brilliant marketers at GE had borrowed the story of Spitteler’s cat for their commercial. But polycarbonate was not the first product made by the GE Plastics division. Rather, it was phenolic. Remember, GE’s core competency was in the electrical industry, where phenolic first made its mark. GE began advancing phenolic chemistry in the late 1920s after Baekeland’s patents expired and sold a material under the trade name Genal until the early 1980’s.
The success of the casein-formaldehyde chemistry took place before Baekeland had his breakthrough with phenolic. But it was this success that reignited interest in Baeyer’s early experiments. And while several chemists were working on this new chemistry simultaneously, it was Baekeland who worked out the system that controlled the considerable explosive force associated with the generation of the by-products from the condensation reaction involved in producing the material. Earlier experimenters had attempted to control the reaction by lowering the temperature to slow things down, and for a time Baekeland followed the same strategy. His breakthrough came when he tried the opposite approach, raising the temperature and controlling the resulting faster reaction by running it in a pressurized vessel, the aforementioned Bakelizer.
The complexity of the phenolic polymerization contributed to Baekeland’s decision to go into the production side of the business rather than make his money by granting licenses. The process was just too complicated for manufacturers without a chemistry background. The Bakelizer was something that would never pass an OSHA inspection. It included an agitator that required electric power. But the nascent electric grid had not reached Baekeland’s area at that point. So, he acquired a steam engine and supplied the steam to the engine using a coal-fired boiler set up in a corner of the laboratory. The steam was then piped across to a garage where the resin manufacturing was done. A fire consumed most of the garage in March of 1909, leading Baekeland to relocate to a chemical works in Perth Amboy, N.J., where a major formaldehyde manufacturer was located.
The first fully synthetic polymer made its mark in electrical insulators, but over the course of the next 30 years it extended its influence into a wide variety of markets that included appliances, office equipment, communications, automotive, aircraft, and weaponry, as well as the more trivial areas of bathroom fixtures and pen barrels. The moldability of phenolic gave birth to the discipline of plastic design. And it fostered other chemistries based on crosslinking with formaldehyde, including urea and melamine. These materials were more easily colored and had better resistance to a long-term effect of electrical current known as tracking.
The first synthetic polymers were thermosets, and they dominated the plastics industry for decades, a far cry from the landscape of our industry today. But the incursion of thermoplastics was already starting and would change things profoundly beginning in the 1930s. We will turn our attention to that part of the story next.
ABOUT THE AUTHOR: Michael Sepe is an independent materials and processing consultant based in Sedona, Ariz., with clients throughout North America, Europe, and Asia. He has more than 45 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting, and failure analysis. Contact: (928) 203-0408 •mike@thematerialanalyst.com
Related Content
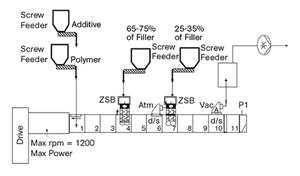
Improving Twin-Screw Compounding of Reinforced Polyolefins
Compounders face a number of processing challenges when incorporating a high loading of low-bulk-density mineral filler into polyolefins. Here are some possible solutions.
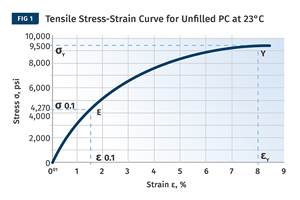
Read MoreThe Effects of Stress on Polymers
Previously we have discussed the effects of temperature and time on the long-term behavior of polymers. Now let's take a look at stress.
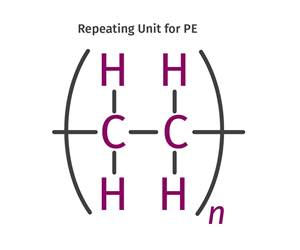
Read MoreThe Fundamentals of Polyethylene – Part 2: Density and Molecular Weight
PE properties can be adjusted either by changing the molecular weight or by altering the density. While this increases the possible combinations of properties, it also requires that the specification for the material be precise.
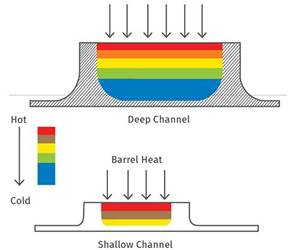
Read MoreThe Importance of Barrel Heat and Melt Temperature
Barrel temperature may impact melting in the case of very small extruders running very slowly. Otherwise, melting is mainly the result of shear heating of the polymer.
Read MoreRead Next
Tracing the History of Polymeric Materials: Phenolics
In this installment we discuss the discovery of Bakelite, the first truly synthetic polymer, known today as phenolic.
Read MoreTracing the History of Polymeric Materials: Celluloid & Film Stock
In this series we delve into a discerning look back into the history of our industry and how we all got here.
Read More
.jpg;width=70;height=70;mode=crop)