Use Cavity Pressure Measurement to Simplify GMP-Compliant Medical Molding
Cavity-pressure monitoring describes precisely what’s taking place inside the mold, providing a transparent view of the conditions under which a part is created and ensuring conformance with GMP and ISO 13485 in medical injection molding.
In a market like medical, robust documentation of process data isn’t a nice-to-have it’s a must-do. (Images: Kistler Group)
Product quality is the overriding priority when it comes to injection molding for the medtech sector. Compliance with statutory requirements and standardization systems such as Good Manufacturing Practice (GMP) is also a fundamental requirement for every market player. But at the same time, global competition is pushing up expectations for production efficiency. The result? Manufacturers are striving not only to achieve reliable, reproducible and documented quality, but also to keep control of the costs involved. Cavity-pressure measurement integrated into the production process can give injection molders an effective instrument that helps them to overcome this dual challenge.
Validated high product quality is an essential condition in medical molding, rather than a competitive advantage or differentiating feature as in other industries.
GMP comprises guidelines on quality assurance that govern the manufacturing processes and environments for a vast range of goods, including medicinal products and medical devices. The goal is a high-quality production process all the way from material procurement to warehouse logistics, which is also key to high product quality. Above and beyond this, quality management that’s compliant with GMP and ISO 13485 should ensure that the regulatory requirements for marketing the products are met. In other words, validated high product quality is actually an essential condition in the medtech industry, rather than mainly a competitive advantage or differentiating feature as is the case in other industries.
The two core elements of GMP are qualification of machinery and plant and validation of processes and methods. The first element requires a planned and documented multistage process to show that plant and equipment are basically suitable for the purpose, and that they do actually function reliably under the conditions prevailing on-site.
Process and method validation also calls for documentation and proof that the processes and methods used will generate reliable and reproducible results, and that the manufactured product will conform to the requirements. To achieve these high standards, especially with regard to documentation, process monitoring based on cavity pressure has repeatedly proven its effectiveness in injection molding. This parameter is the most informative process value in injection molding, because it gives users a completely transparent view of the process to create a molded part. It goes without saying that this helps users to meet their documentation obligations. As an added benefit, cavity-pressure monitoring can simplify process validation during machine setup and make it easier to optimize production processes, with zero-defect production based on quality prediction models as the ultimate goal.
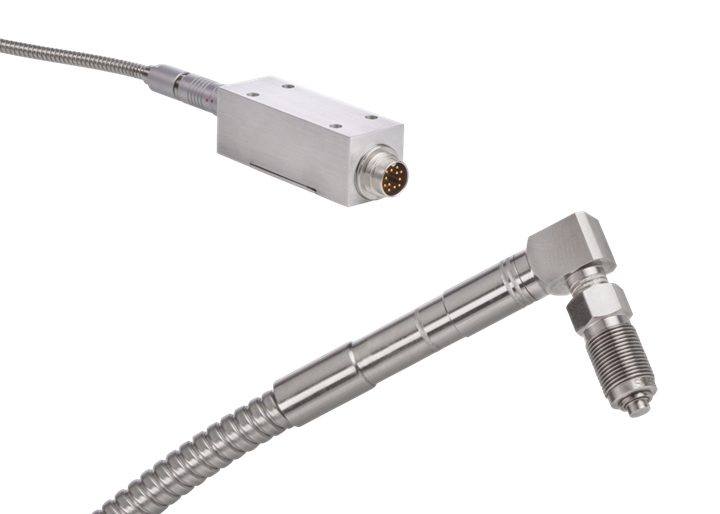
In-cavity sensors, like this 4004A piezoresistive melt-pressure sensor from Kistler, can provide highly accurate data in support of ISO and GMP documentation.
Correlation with Part Quality
What makes cavity pressure such an informative and highly relevant value? As a process parameter, it is captured directly in the cavities with the help of pressure sensors. It precisely describes the processes taking place in the cavities, thus providing a transparent view of the conditions under which the part is being created throughout the injection molding process. Essential advantages include the specific quality-related features of the part such as dimensional accuracy, surface characteristics, weight and degree of molding, which can be attributed to the cavity-pressure profile during the injection, compression and holding phases.
It follows that the cavity-pressure profile can be considered as a fingerprint of the quality of the specific part that is currently being produced. It provides the basis for precise statements about optimal process parameters throughout the production process. Good parts can already be differentiated from bad ones while production is still in progress. For this purpose, the upper and lower limits of the key values are determined for validation of the respective process parameters. If the value determined from the curve then goes beyond the defined process window during production, the part is classified as bad and will be separated automatically. The result is that only good parts continue further into the value chain. This is not the only benefit, however. The characteristic values obtained can also be used as inputs for statistical process control (SPC). See Fig. 1.
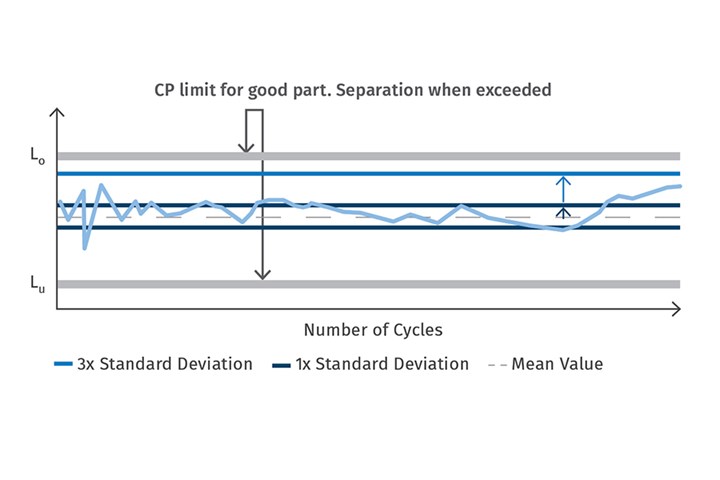
With the help of cavity pressure measurement, good and bad parts can be automatically differentiated and separated during production. The data can also be used as an input for statistical process control to indicate how robust a process is.
Photo Credit: Kistler
Validation based on cavity-pressure measurement yields even more advantages. For example, significantly less effort is required compared with methods that take account exclusively of machine process parameters. This is because the processes in the machine cannot adequately describe the formation of the molded part in the cavity, so correlation with part quality is difficult. To achieve consistent part quality, the machine parameters then have to be adapted to new conditions, such as changes in material behavior when batches of different resins are processed. If the fluctuations are so pronounced that the tracked settings are outside the previously validated process window, the process needs to be validated again, which involves a series of time-consuming trials. This can all be made far easier by using cavity-pressure measurement, so the correlation between measured values and quality characteristics is known (Fig. 2).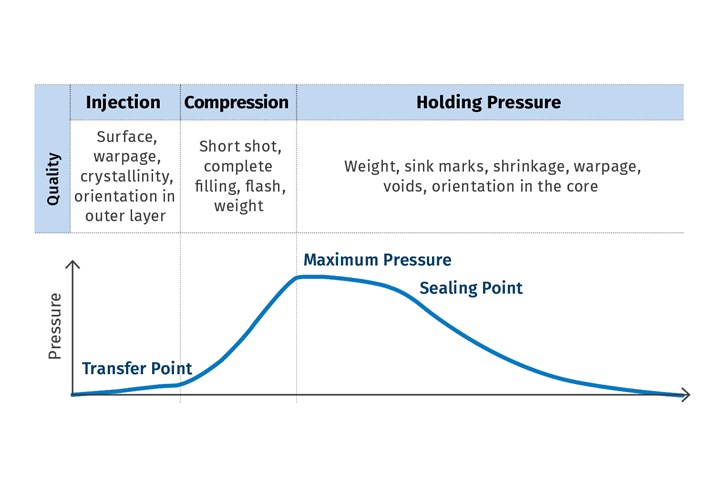
The cavity pressure values measured during the various phases of the injection molding cycle correlate to the part’s quality characteristics.
Photo Credit: Kistler
Smart Combo of Measurements and Statistics
A process-monitoring solution based on cavity-pressure measurement consists of sensors that are highly precise but also robust, paired with a process-monitoring system with software that optimizes setting up a production process; provides efficient, automated documentation of the test plans (Design of Experiments, or DOE); and performs the corresponding process analyses.

By pairing sensors with a process monitoring system, like Kistler’s ComoNeo unit, molders can gather data and optimize processes.
Operators can take a decisive step towards zero-defect production by using these tools to determine the expected quality characteristics from the measured values, with no need to expend effort on measuring the parts. Thanks to this solution, a part’s quality can be predicted even before it is manufactured, including the necessary documentation.
Preventive Quality Assurance
Operating a process-monitoring system in combination with process-optimizing software also reduces the effort required during process analysis and development. To find a stable process window on a qualified machine in the final phase of process development, coordinated DOEs need to be created and executed. The purpose of the DOE with varying machine parameters is to achieve variance of the process conditions represented by the cavity pressure so as to cover the process window.

Cavity-pressure monitoring can provide zero-defect production, preventive quality assurance, and minimized need for process revalidation. (Photo: Cleanroom molding at Caplugs Medical)
Mathematical correlation of the measurement values with the continuous, attributive quality characteristics that are measured afterwards supplies a model for ongoing prediction of the quality of each part produced. With some programs, the model attains a very high degree of accuracy, thanks to special algorithms and the use of machine learning. This makes it possible to predict part quality during production, and also to separate parts that are out of tolerance. The benefits: Required quality can be guaranteed and testing costs can be drastically reduced, giving injection molders the conditions to safeguard their competitive edge.
About the Author Robert Vaculik
Robert Vaculik works as a consultant for the plastics industry. Before founding his independent consultancy in 2022, he was head of the Plastics Business Unit at Kistler Group. After studying mechanical engineering at RWTH Aachen University, he completed a Ph.D. in injection molding process control at IKV Aachen and worked at Mann + Hummel, Dynamit Nobel, ITW Engine Components and Montaplast, among others. Contact: 248-668-6900; kistler.com.
Related Content
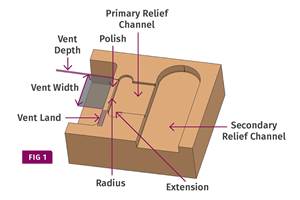
Back to Basics on Mold Venting (Part 2: Shape, Dimensions, Details)
Here’s how to get the most out of your stationary mold vents.
Read MoreThree Key Decisions for an Optimal Ejection System
When determining the best ejection option for a tool, molders must consider the ejector’s surface area, location and style.
Read MoreThe Effects of Time on Polymers
Last month we briefly discussed the influence of temperature on the mechanical properties of polymers and reviewed some of the structural considerations that govern these effects.
Read MoreThe Effects of Stress on Polymers
Previously we have discussed the effects of temperature and time on the long-term behavior of polymers. Now let's take a look at stress.
Read MoreRead Next
Getting Started with Medical Molding: First Consider the ‘Four E’s’
Global medical molder Nypro provides first-hand guidance on what you need to get into the medical molding business.
Read MoreSo You Want to Be in Medical Plastics? Ask Yourself These Questions First
A medical custom processor cautions those in the field, or wanting to get into it, about regulatory trends that add complexity and risks to an already demanding market sector. Understanding the answers to four key questions can help clarify the issues.
Read More