A Gel By Any Other Name...
Gels…unmelt…contaminants…inclusions…whatever you call them, you want to avoid them. Proper maintenance and good housekeeping—lids on boxes, closed hopper doors, core-free reclaim systems—go a long way towards defect-free product.
In one of my previous lives at The Dow Chemical Company, I ran an optical microscopy lab in the Dow Pack Studios facility in Freeport, Texas. One of our tasks was to analyze different inclusions to determine the type and source. This analysis would include making a slide and placing the sample on a hot stage where it would be heated under a controlled manner to melt the polymer away from the inclusion and see how the inclusion responds to heat. The hot stage would be placed under an optical microscope usually at 50× magnification.
I use the term inclusion rather than gel because there seems to be some debate as to what the definition of a gel is. Some say, “It’s not a gel, it’s an un-melt”, or “It’s not a gel, it’s a contaminant”. A quick Google search for the definition of a polymer gel will get you the definitions: gelatin, jelly, bath gels, and others.
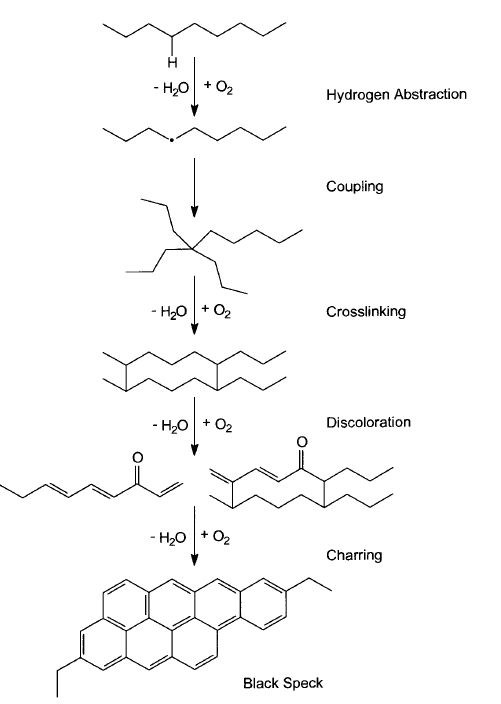
For our purposes here, the most direct definitions of polyethylene gels usually include language around cross-linking or covalent bonding. This is where the debate usually begins. Scientifically, PE gels are large polymer molecules where bonding has occurred between molecules usually involving other mechanisms like oxidation. However, outside of analyzing these “gels” under a microscope and observing their response to heat, it is often very difficult to determine the type of inclusion with the naked eye.
Obviously, if the gel is not clear and has some color to it, (i.e. yellow, brown, or amber) it can easily be determined to be oxidized. But is it cross-linked? What about carbon, where did it come from?
Personally, I think there is a better way to define a gel that works for most film and many rigid applications. A practical definition of a gel needs to satisfy these three conditions:
- It forms a point of stress in your product; and
- It will cause premature failure; and
- You don’t want it to be there.
Let’s walk through a couple of examples and see if this definition holds water. Case one is a film that was sent to Dow’s Pack Studios in Freeport for gel analysis. Upon receiving the film, the customer had circled several spots with a marker. The films samples were placed under an optical microscope at 50× magnification and inside each of the ink circles was a small “gel.”
From a practical application standpoint, gels are anything in our product that causes stress, potentially causing failure, and we don’t want them present.
Some were large—approaching 25 µm—and others were only a few microns across. The gels were cut from the film and slides prepared and placed on a hot stage heater. The temperature was increased under a controlled manner and different techniques used to monitor the gel as the temperature reached the melting point of the material around the “gel.”
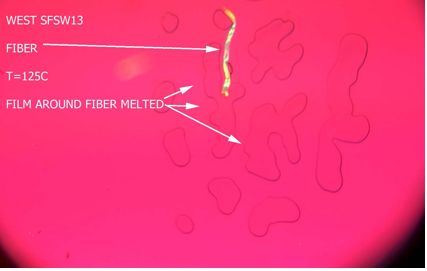
As the “gel” melted, a small fiber was left behind. Upon closer inspection at higher magnification the fiber appeared to be brown in color and was attributed to cardboard. The data was communicated to the customer and it was determined that a cardboard film core was accidentally shredded with the film scrap used as re-claim.
Case two again included a film with several circles marked on it and each of those containing a “gel.” The same procedure above revealed a very straight, sharp, blue, man-made fiber. Upon investigation with the customer at their facility, it was concluded that the source of the fiber contaminant was the fact that the resin boxes were stored open, with the lids off and positioned under the air-condition intakes which included blue fiberglass filters. So, for both case one and two we have fiber contamination that are forming a stress point, potentially causing premature failure, which the customer does not want in there.
Case three was a film with “gels” again analyzed as above. This time as the temperature of the hot stage approached the melting point of the material around the gel, the film melted away and then within just a few degrees the “gel” melted as well. At this point, the temperature was held steady and different techniques were employed to look for evidence of cross-linking, and none was found. The sample was then allowed to cool back to room temp and the gel did not re-form. This particle was classified as an un-melt and steps were taken with the customer to optimize the system to prevent the reoccurrence of the un-melts. Again, in case three, we had an inclusion causing a stress point, which could potentially cause premature failure, and we do not want it to be there.
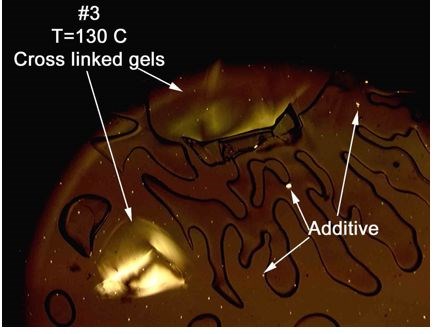
Case four: we had a film sample from a customer who again had circled the inclusion. In this instance, many of the gels had a slight yellowish color that could be seen by the naked eye, indicating a degree of oxidation for the gel. Upon analysis, the material melted away from the gel, and the gel was allowed to heat up much higher. The temperature ramp was paused, and different techniques were used to look for signs of cross-linking via flow patterns upon stressing and it was found. Again, we had an area of potential stress, which could potentially cause failure, and we don’t want it.
So, the gel in case number four was a cross-linked gel. “But wait, you said it was oxidized,” you might protest. That is true. See, oxidized gels have a life cycle. Cross linked gels can be formed in the presence of, or absence of oxygen. When oxygen is present, oxidation occurs. Then, given enough time, cross-linking will follow. The gel can then be classified as cross-linked and slightly oxidized. The “gel” continues to react and pick up color through development of conjugated double bonding.
At this point, the gel is crosslinked and slightly oxidized, and usually yellow. Next, the level of conjugation continues to increase, and the color intensifies. These gels are cross-linked, oxidized and usually amber in color. As the level of conjugation becomes more complete, the gels become cross-linked, oxidized and brown. Finally, the gels become completely carbonized and black.
Is it a gel if it’s not crosslinked? Scientifically speaking, no. So, only gels are crosslinked, and they form a stress point that could cause a failure, and we don’t like them. True, but so do un-melts, fiber contaminants, bug wings, pieces of cotton gloves, parts of gaskets from resin hoppers, and any of the other oddities I have seen show up in film and rigid part analysis. From a practical application standpoint, gels are anything in our product that causes stress, potentially causing failure, and we don’t want them present.
Either way gels, inclusion, unmelts, contaminants, etc. cause problems. Regardless of what we call them, the best solution to the question is to avoid getting any of them in the first place. Proper maintenance, good housekeeping, keeping lids on boxes, doors closed on hoppers, and cores out of reclaim systems can go a long way toward producing a defect-free product.
ABOUT THE AUTHOR: Wes Hobson is a senior engineer in The Dow Chemical Co.’s Performance Plastics Technical Service and Development group in Freeport, Texas. He has 22 years of experience focusing on cast and blown film and sheet extrusion. Hobson is currently application development leader in Dow’s Industrial and Consumer Packaging group, focusing on stretch film. He has a Master’s Degree in Packaging Science from Michigan State University and is a member of ISTA and SPE. Contact: 979-238-1610; jhobson@dow.com; dow.com.
Related Content
Got Streaks or Black Specs? Here’s How to Find and Fix Them
Determining the source of streaking or contamination in your molded parts is a critical step in perfecting your purging procedures ultimately saving you time and money.
Read MoreBozzelli’s Guide To Specifying a Dryer
Here's a list of 17 things to do when looking for new drying equipment.
Read MoreThe Effects of Stress on Polymers
Previously we have discussed the effects of temperature and time on the long-term behavior of polymers. Now let's take a look at stress.
Read MorePlastics Technology Year in Review: Your Favorite Reads of 2024
A year-end review of the top stories showcasing industry trends, advancements and expert insights. Revisit the articles that captured the attention of the plastics community.
Read MoreRead Next
People 4.0 – How to Get Buy-In from Your Staff for Industry 4.0 Systems
Implementing a production monitoring system as the foundation of a ‘smart factory’ is about integrating people with new technology as much as it is about integrating machines and computers. Here are tips from a company that has gone through the process.
Read MoreMaking the Circular Economy a Reality
Driven by brand owner demands and new worldwide legislation, the entire supply chain is working toward the shift to circularity, with some evidence the circular economy has already begun.
Read More