Novel Catalyst for Converting Carbon from Hard-to-Abate Sectors
RenewCo2’s technology can transform carbon into feedstock for carbon-negative, plastics monomers, such as MEG and ethanol.
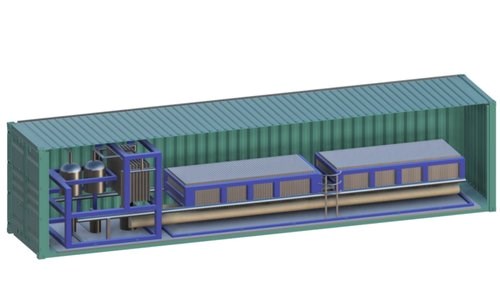
Co2RR unit and container
Photo Credit: RenewCO2
We were recently contacted by new startup RenewCO2, which announced its spin-out from Rutgers University, securing an exclusive licensing to scale its novel catalyst technology to convert carbon from hard-to-abate sectors and transform it into a feedstock for carbon-negative, plastic monomers at a fraction of the cost of plastics derived from fossil sources.
Founded by immigrants Dr. Karin Calvinho and Dr. Anders Laursen out of one of the leading catalyst laboratories in the US, the startup has raised over $10 million in venture investment and grant funding, including a seed round of over $2 million led by Energy Transition Ventures. RenewCO2 expects to begin supplying its eCUT electrolysis systems to customers by 2025.
The RenewCO2 team has extensive experience in catalysis, electrolyzers, electrochemistry, chemicals and materials science. The company has repeatedly demonstrated its novel catalyst can essentially mimic nature's route to reducing CO2 with efficiencies that far exceed those of natural systems by chemically transforming carbon dioxide into chemicals at unprecedented selectivity and high energy efficiency in a single step. The low-cost catalyst has also received broad peer-review attention and several awards, including features in The Royal Society of Chemistry’s Energy & Environmental Science journal and the Journal of the American Chemical Society.

RenewCO2’s electrolyzer
Photo Credit: RenewCo2
Said Karin Calvinho, co-founder and chief technology officer of RenewCO2, also a DOE Chain Reaction Innovations Fellow at Argonne National Lab. "When I came to the United States to pursue my graduate degree, I was sure I wanted to advance research in catalysis for renewable energy. When we discovered our catalyst, I knew we had the opportunity to reverse global carbon dioxide levels at a mass scale. With the potential to decarbonize chemicals, refining, power generation, biofuel production, and many other CO2-emitting processes, we are determined to change the way polymers are made by displacing fossil carbon resources while using renewable energy sources and making sustainable products."

Karin Calvinho (white lab coat) and interns Simon Li and Gabrielle Solymosy.
Photo Credit: RenewCO2
With climate change rapidly intensifying, the petrochemical and chemical industries' decarbonization efforts and customer demand for zero carbon products are driving a focus on Carbon Capture and Utilization (“CCU”) technologies, including recently expanded support for CCU in the US Inflation Reduction Act. Energy and industrial CO2 point sources and direct air capture projects are ideal for supplying RenewCO2's modular, scalable eCUT electrochemical conversion process. The RenewCO2 catalyst technology benefits from falling costs and advancements in electrolysis and renewable power, making it both modular and easily scalable into a lower-cost solution than fossil feedstocks. Large CO2 streams can be converted selectively into a range of demonstrated products in a single unit, including the precursors for various plastic materials such as monoethylene glycol (MEG), methylglyoxal, and furandiol. Such plastics and polymers are foundational to manufacturing electronics, appliances, plastic bottles, food containers, textiles, and more.
According to co-founder and CEO Anders Laursen, RenewCO2’s eCUT process goes from CO2 to valuable products in a single energy-efficient step, not just conversion to CO or syngas. “By harnessing CO2 as the feedstock and building on the rapidly growing field of electrolysis, we can provide companies with an extremely sustainable replacement to fossil fuels when it comes to creating plastics."
The key innovation of the RenewCO2 eCUT process is the use and design of catalysts based on abundant metals to achieve more than 90% energy efficiency for reducing CO2 to multi-carbon products (e.g., monoethylene glycol, methylglyoxal,and furandiol). The RenewCO2 eCUT platform can selectively produce multiple products with a change in catalyst and reaction conditions, making the expansion to secondary markets rapid once demonstrated in the beachhead market.

Anders Laursen synthesizing catalyst.
Photo Credit: RenewCO2
Transition metal phosphides unlock a new mechanism that is responsible for reported gains in energy efficiency. Through a combination of computational chemistry and experiments, the research team has shown that this class of catalysts forms hydrides at the surface, converting CO2 directly to HCOO-(formate), and reducing it to formaldehyde. Carbon-carbon coupling of formaldehyde, as with most other aldehydes, is both kinetically and thermodynamically favorable, allowing for a reaction that produces longer carbon chains with very little energy consumption. This mechanism has been verified by other research groups.
While the reaction mechanism involves several steps, they all occur directly on the catalyst surface in a fraction of a second. The process occurs in a single-step electrochemical reactor where the inputs are water, electricity, and CO2, and the output is the product, e.g., monoethylene glycol and pure oxygen. By judiciously designing changes in the catalyst's atomic structure (crystal structure), they can tune the selectivity between different products, unlocking rich chemistry that can lead to the synthesis of a variety of commodity and specialty chemicals.
Monoethylene glycol (MEG), key feedstock in the production of PET, is the chosen beachhead market because it combines favorable process economics, high customer interest, and the potential to significantly reduce CO2 emissions due to its large production volume. Additionally, the incumbent petrochemical MEG process generates high-purity CO2 as a by-product. This CO2 can be used with little to no purification as the feedstock for the RenewCO2 eCUT-MEG process, which lowers the barrier for technology demonstration as eCUT can then be implemented modularly as an add-on process.
Formic acid (FA) is the team’s second target product because of its significant current use in agriculture (a high CO2 impact industry), environmentally friendly deicers, as well as its future use as a liquid hydrogen carrier, an application with the potential to displace olefins for hard-to-reach locations with huge potential CO2 emissions savings.
The main technical challenges for the commercialization of CO2-to-liquid product technologies are related to the size of the electrolyzer stacks and the power infrastructure required to produce chemicals at the commodity market scale. However, advances in CO2 electrolyzer manufacturing at scale for MEG and FA will be rapidly translatable to other liquid products, such as propylene glycol, ethanol, and methanol.
Previously, the company had announced a $225,000 grant from the National Science Foundation and a $1.15 million in research and development funding from the Department of Energy 0 (DOE) for its various solutions in utilizing carbon emissions to promote decarbonization in the production stages of the chemical industry. Most recently, it announced an additional $200,000 from the DOE with Rutgers for conversion of CO2 to ethanol. The company has won $8M in grants and contracts for eCUT.
Related Content
How to Optimize Your Molds and Hot Runners for Processing Bioresins
Demand for bioresins is growing in molded goods, particularly as a sustainability play to replace fossil-fuel based materials, but these materials are not a drop-in replacement for traditional materials. Molds and hot runners need to be optimized for these materials.
Read MoreAvantium and U.S.’s Origin Materials Aim to Accelerate Production of FDCA and PEF
The partnership aims to bring together both companies’ technology platforms to produce FDCA from sustainable wood residues on an industrial scale.
Read MoreBest Practices for Purging PHA and PHA/PLA Blends
Because bioplastics are processed at lower temperatures, purging between jobs requires a different process and purging agents than those applied for traditional resins.
Read MoreHonda Now Exploring UBQ’s Biobased Material Made from Unsorted Household Waste
UBQ is aiming to expand its reach for more sustainable automotive parts as well as non-automotive applications.
Read MoreRead Next
See Recyclers Close the Loop on Trade Show Production Scrap at NPE2024
A collaboration between show organizer PLASTICS, recycler CPR and size reduction experts WEIMA and Conair recovered and recycled all production scrap at NPE2024.
Read MoreLead the Conversation, Change the Conversation
Coverage of single-use plastics can be both misleading and demoralizing. Here are 10 tips for changing the perception of the plastics industry at your company and in your community.
Read More