Waste Plastics Must D-I-E
New research publications describe dehydrogenation, isomerization, ethenolysis as a pathway for polyethylene recycling.
Consumer concern over plastic waste has driven demand for recycled plastic upwards, while supply has struggled to keep pace. Improved collection infrastructure and changing behaviors can have immediate impact on the achievement of recycled content goals. In the long term, the development of new recycling technologies can contribute by expanding the breadth of collected recyclables that can be processed into useful material.
Recently published research describes methods for using waste polyethylene to create propene, the building block monomer for polypropylene, possibly facilitating recycling of the most commonly produced plastic into the second-most.
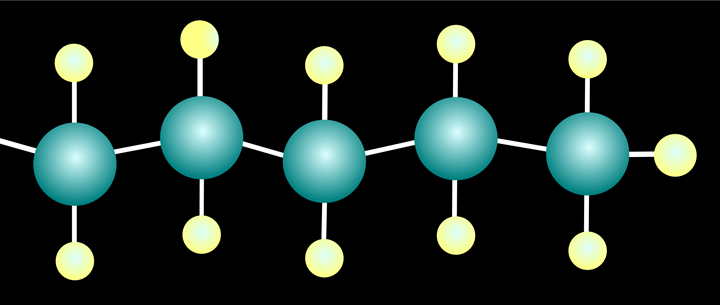
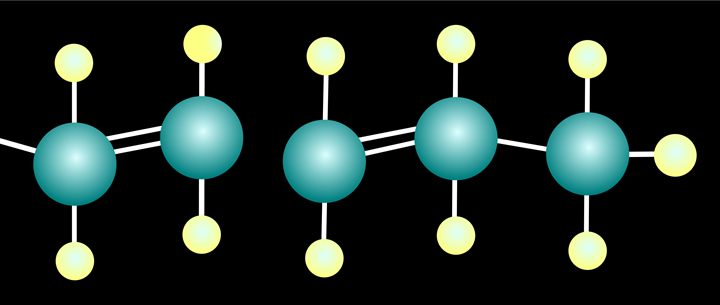
A very simple carbon chain, saturated with hydrogen.
Producing high-value plastic feedstock from PE requires breaking the strong carbon-carbon bonds that form the polymer backbone. Currently, the most common process for doing this is pyrolysis, smashing this bonds with extreme heat. Pyrolysis can create oils and new monomer from plastic wastes, but the high temperature requirements result in a large greenhouse-gas footprint.
Many alternatives are being investigated by companies and by academic researchers. Variations on a particular approach have generated some buzz in recent weeks. It starts with the use of a catalyst to pull hydrogen atoms off the carbon chain, creating a single double bond (dehydrogenation).
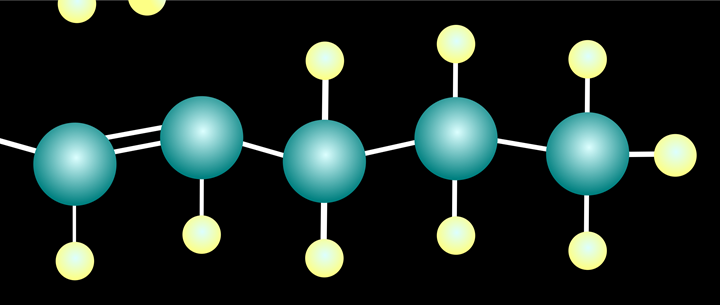
Dehydrogenation leaves PE with at least one double bond.
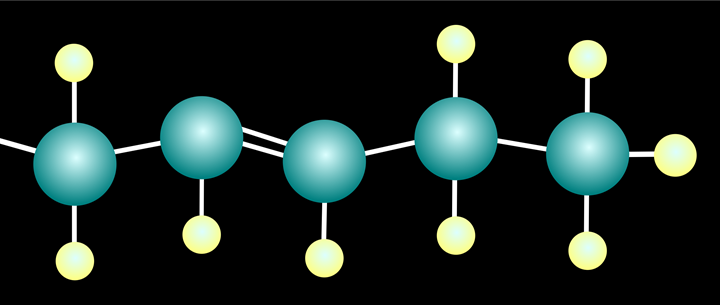
Isomerization reactions change the position of the double bond.
Finally, the carbon chain is cut in two by reaction with ethylene, forming a smaller hydrocarbon. I’ve chosen to illustrate a simple case here that is simple and gives us exactly what we want: propene. Of course, in a real process propene would be just one of many products, and reducing that variation is one of the challenges.
Here the propene molecule has been split off from the chain by ethylene.
In the journal Science, a group from UC Berkeley (Conk et. Al) described the results of using various catalysts to break down both virgin and postconsumer HDPE and LDPE into propene. The reported yields were up to 80%, and the processing temperatures much lower than pyrolysis.
In the Journal of the American Chemical Society, a collaboration between scientists at the University of California, the University of Illinois at Urbana-Champaign, and the Dow Chemical Company published the results of their research, describing tandem processes to convert PE into propene monomer, conducting dehydrogenation, isomerization, and ethenolysis simultaneously. A preliminary carbon footprint analysis showed a modest reduction in greenhouse gas emissions relative to conventional propylene production. But, as the authors point out, much larger emissions reductions could be achieved by using renewable electricity and by sourcing ethylene sustainably.
Also in the Journal of the American Chemical Society, Zichittella et. Al recently described the use of cobalt-based catalysts for dehydrogenation of PE and PP to create propane. Itself a useful hydrocarbon, propane can also be used to create new polyolefins. Colbalt-based catalysts were selected to avoid the use of precious metals like platinum, while generating the desired low-weight hydrocarbons without too much methane. The research team concluded that colbalt combined with a zeolite performed the best in terms of weight selectivity and may have future utility in the processing of postconsumer HDPE, PP, and LDPE.
Each of the three studies expands the available knowledge, looking at different combinations of catalysts and conditions to try and address the problems of cost and energy while achieving high yield of valuable recyclate from PE that has reached the end of its useful life.
It may be some years before we see a process like these at work on an industrial scale, but in the meantime I will be interested to watch and learn as chemists try out different approaches. There is a lot of polyethylene in the world that is no longer usable, likely to be a whole lot more in the very near future, and turning it in to something as useful as new PP would be no small feat.
Related Content
Prices Flat-to-Down for All Volume Resins
This month’s resin pricing report includes PT’s quarterly check-in on select engineering resins, including nylon 6 and 66.
Read MoreFundamentals of Polyethylene – Part 3: Field Failures
Polyethylene parts can fail when an inappropriate density is selected. Let’s look at some examples and examine what happened and why.
Read MoreFundamentals of Polyethylene – Part 6: PE Performance
Don’t assume you know everything there is to know about PE because it’s been around so long. Here is yet another example of how the performance of PE is influenced by molecular weight and density.
Read MoreIn Sustainable Packaging, the Word is ‘Monomaterial’
In both flexible and rigid packaging, the trend is to replace multimaterial laminates, coextrusions and “composites” with single-material structures, usually based on PE or PP. Nonpackaging applications are following suit.
Read MoreRead Next
Making the Circular Economy a Reality
Driven by brand owner demands and new worldwide legislation, the entire supply chain is working toward the shift to circularity, with some evidence the circular economy has already begun.
Read MoreLead the Conversation, Change the Conversation
Coverage of single-use plastics can be both misleading and demoralizing. Here are 10 tips for changing the perception of the plastics industry at your company and in your community.
Read MoreBeyond Prototypes: 8 Ways the Plastics Industry Is Using 3D Printing
Plastics processors are finding applications for 3D printing around the plant and across the supply chain. Here are 8 examples to look for at NPE2024.
Read More