Carbon 3D Prints Test Swabs and Face Shields in Response to the Coronavirus
Resolution Medical is among the first manufacturers to use the Carbon Platform to 3D print a conformal lattice swab, creating a medical device for the testing of COVID-19.
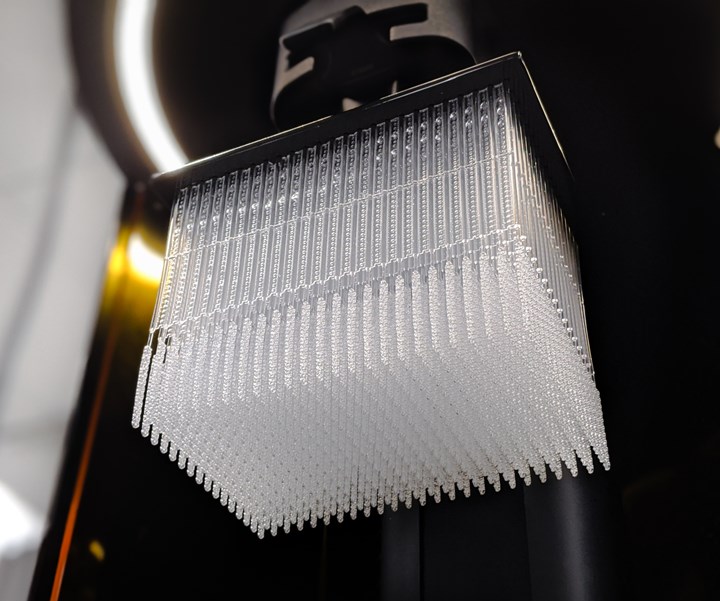
The Carbon Digital Manufacturing Platform is now being used to produce 1M+ nasopharyngeal swabs per week by Resolution Medical, an in vitro diagnostic and medical device manufacturer.
It’s been so impressive to see how manufacturers have responded to the COVID-19 crisis by producing equipment to protect healthcare workers and increase testing capacity.
Resolution Medical, an FDA registered, in vitro diagnostic and medical device manufacturer, announced the launch of The Resolution Medical lattice swab, Crafted with Carbon technology, Resolution Medical has already sent the new 3D printed nasopharyngeal swabs for COVID-19 testing to healthcare organizations and is able to provide swabs to others in the U.S. who order on its website. Manufactured using Carbon Digital Light Synthesis (DLS) technology and KeySplint Soft Clear material for Carbon printers from Keystone Industries, the lattice swab is classified by the FDA as a Class I 510(k) Exempt in vitro diagnostic medical device.
Patient testing swabs are among the most critical medical supplies needed by healthcare providers for COVID-19 testing. Expanding the availability of testing supplies, and thus testing frequency, is a vital part of the timely identification of COVID-19 patients and helping to curb the pandemic’s spread. The new Resolution Medical lattice swab exhibits a conformal lattice design made with Carbon’s Lattice Engine software. The hollow structure of the lattice is designed for specimen collection efficiency, with a geometry that is also flexible to promote functionality and comfort for patients. The product is now undergoing clinical evaluation by clinicians at multiple institutions, including Beth Israel Deaconess Medical Center (BIDMC), a teaching hospital affiliated with Harvard Medical School, and Stanford Medicine.

“We are proud to be collaborating with digital manufacturing company, Carbon, to produce the lattice swab,” said Shawn Patterson, founder and president of Resolution Medical. “We have worked together urgently to get this product into the hands of healthcare workers to help address immediate needs for increased COVID-19 testing.”
“At scale, we plan to supply over 1 million swabs per week.”
To produce the lattice swab, Resolution Medical works with Carbon’s network of dental labs and production partners, as well as its internal printing team, who use Carbon M2 printers and Keystone Industries’ KeySplint Soft Clear material, indicated for the fabrication of orthodontic and dental appliances such as mouthguards, night guards, and snoring appliances in the US, Canada, EU, Australia, and New Zealand. The swabs, which are biocompatible and autoclavable, are currently printed hundreds at a time with a unique serialization present on each strip to facilitate traceability.
“Triggered by the COVID-19 pandemic, Carbon’s engineers and material scientists quickly sprung to action to identify the KeySplint Soft Clear material as having the right balance of properties to make a soft, flexible swab with appropriate strength that could be printed with precision using the Carbon M2 at 75 micron pixels,” said Joseph DeSimone, co-founder and executive chairman at Carbon.
Collaboration
In developing, testing, and refining the lattice swabs, the team collaborated with a coalition of researchers led by clinical pathologist Ramy Arnaout, MD, PhD, associate director of the clinical microbiology laboratories at BIDMC and assistant professor of pathology at Harvard Medical School. His group performed clinical assessments of the swabs based on design, materials testing, usability, and a variety of human factors; collection sufficiency of biological samples; and PCR compatibility using their in-house commercial COVID-19 test. Leading an open and public process, Dr. Arnaout’s group tested and provided feedback on over 160 designs, materials, and prototypes from over a dozen manufacturers, resulting in a decision to launch an ongoing clinical trial of the lattice swabs.
“Resolving the national shortage of nasopharyngeal testing swabs is critical to the global fight against COVID-19," Dr. Arnaout said. "We have brought together a multi-disciplinary team of scientists, academics, and industry partners in a shared effort to resolve this crisis and have begun a clinical trial of lattice swabs and other prototypes here at Beth Israel Deaconess Medical Center.”
Carbon and Resolution Medical also collaborated with Kit Parker, PhD, a lieutenant colonel in the United States Army Reserve and a professor of bioengineering and applied physics at Harvard University, who played a key role in convening and coordinating efforts, as well as assessing the mechanical properties of various swabs submitted by several providers.
Additionally, Carbon and Resolution Medical have been working with a team of faculty and laboratory and nursing staff at Stanford Medicine, including Ryan Van Wert, MD, clinical assistant professor of medicine; Christina Kong, MD, medical director of the Pathology and Clinical Laboratory; and Jennifer Fralick, administrative director of Laboratory Services. The 3D Printing for COVID-19 Taskforce at Stanford Medicine has been led by Sridhar Seshadri, PhD, Chief Administrative Officer, Destination Service Lines.

adidas and Carbon are producing 3D-printed face shields.
3D printed face shields
adidas and Carbon are also rapidly producing 3D-printed face shields during the global COVID-19 (coronavirus) pandemic. Carbon is currently producing more than 18,000 face shields a week, after shifting all manufacturing at its California facilities last month to focus on developing medical supplies for first responders and healthcare professionals. Carbon is also enabling the production capacity of 50,000 face shields per week across its global network of customers and partners who are addressing needs in their respective local areas.
Carbon is working to facilitate local-for-local, on-demand production of these face shields to protect healthcare professionals and first responders on the COVID-19 frontlines. Healthcare workers at Stanford Hospital and Kaiser Permanente have provided Carbon with positive feedback on these face shields.
One of Carbon’s dental customers, Candid, has diverted its manufacturing operations to produce face shields. Candid has the goal of producing thousands of face shields over the next few weeks.
Carbon is also working with its global network of customers and partners, as well as other 3D printing companies such as HP, to address this medical supply shortage.
For more about Carbon’s work in this area, check out the video below:
Related Content
NPE 2024: Additive Manufacturing Assisting, Advancing Plastics Processing
Exhibitors and presenters at the plastics show emphasized 3D printing as a complement and aid to more traditional production processes.
Read MoreInsight Polymers & Compounding Unveils New Conductive Products Line
The new conductive products line will also be produced for injection molding and extrusion.
Read MoreBusiness Slowing? There's Still Plenty of Stuff to Do
There are things you may have put off when you were occupied with shipping parts to customers. Maybe it’s time to put some of them on the front burner.
Read MoreAdditive Technologies for Injection Mold Tooling Ride Tailwinds
NPE2024: Lowering barriers to additive manufacturing adoption in toolmaking.
Read MoreRead Next
3D Printing, Silicon Valley Style
Carbon’s technology seeks to disrupt the 3D-printing industry for good.
Read More3D Printing Helps Keep Production Going During the Coronavirus Crisis
Formlabs and Essentium both discuss how their companies are responding to the COVID-19 crisis and how 3D printing can help eliminate potential supply chain disruption.
Read MoreHP is 3D Printing Parts to Help Battle the Coronavirus Pandemic
Many 3D design files to be made freely available to accelerate critical parts production.
Read More


























