Additive Manufacturing: First High-Purity PVDF 3D Printing Filament
Based on Arkema’s Kynar PVDF, Nile Polymer’s new filament is designed for large parts without dimensional warping.
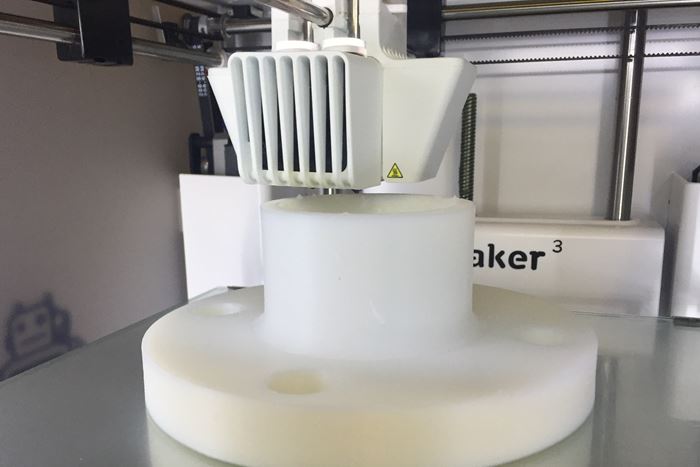
What appears to be the first high-purity PVDF filament for 3D printing of prototypes and small production runs is newly available from Nile Polymers, Centerville, Utah. This innovative processor specializes in providing fluoropolymer products used in the chemical, biopharma and semiconductor industries. All based on Kynar PVDF from Arkema, King of Prussia, Penn., the company’s products include Strong-Ty cable ties, Purisan mixers, and Fluorinar 3D printing filaments. Only limited by print bed size, Fluorinar-C can be printed by FFS/FDM processes and without dimensional warping.
Says president and founder Howard Fisher, “With more than 50 years of industrial use, Kynar PVDF continues to be recognized for its excellent chemical resistance, UV and ionizing resistance, non-flammability and high-temperature performance. With our family of products, Nile Polymers builds upon this product Kynar PVDF legacy.”
This newest addition to the Fluorinar portfolio—Fluorinar-C copolymer filament is designed for large parts. Says Arkema sales engineer Gene Alpin, “This new filament stays secured to the build plate during printing and comes with sll the certifications that is expected with Kynar PVDF, such as USP Class VI, NSF 51/61, and FDA certifications. This is the first warp-resistant material to offer these certifications.” He sees it primarily for use of prototypes and small production runs of parts such as as clamps, fittings, and vessel components used in several single-use biopharma applications.
Fluorinar-C is available in 1000 g spools with either 1.75 mm or 2.85 mm diameter with a +/- 0.05 mm tolerance. Nile Polymers is offering a 5-meter sample of either size filament to those interested in trialing Fluorinar-C.
Related Content
-
Use Cavity Pressure Measurement to Simplify GMP-Compliant Medical Molding
Cavity-pressure monitoring describes precisely what’s taking place inside the mold, providing a transparent view of the conditions under which a part is created and ensuring conformance with GMP and ISO 13485 in medical injection molding.
-
Baxter to Scale Up PVC Intravenous Bag Recycling Program
Successful pilot program with Northwestern Medicine will expand to additional units and health systems.
-
Medical Molder, Moldmaker Embraces Continuous Improvement
True to the adjective in its name, Dynamic Group has been characterized by constant change, activity and progress over its nearly five decades as a medical molder and moldmaker.