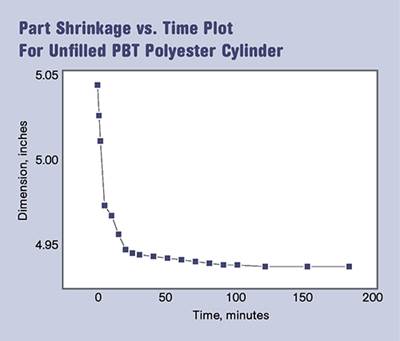
Dimensional Stability after Molding—Part 3
Any process that involves melting and re-solidifying a polymer involves a compromise between achieving the perfect structure and producing a part that can be sold at a price that the market is willing to pay.
Amorphous polymers are generally considered to be a molder’s insurance policy against the problems of dimensional stability.
Amorphous structures undergo a smaller and more predictable change in volume as they cool from the melt to the solid, making them easier to mold to close tolerances. If you study the shrinkage behavior of a part molded in an amorphous material, you will also find that the part achieves stable dimensions in a shorter period of time. However, over time amorphous polymers also undergo a slow and subtle structural change that can result in continued shrinkage. This process is known as physical aging and it was not well understood in polymers until the 1970s.
Any process that involves melting and re-solidifying a polymer involves a compromise between achieving the perfect structure and producing a part that can be sold at a price that the market is willing to pay. Optimal structural stability is achieved by allowing the polymer chains in a system to reach their ideal configuration in terms of spatial separation. In a semi-crystalline material, as we have already discussed, this means attaining an optimal degree of crystallinity. In amorphous polymers, where no significant level of crystallinity is obtained, the ultimate objective is something called thermodynamic equilibrium. In both cases this involves allowing the polymer chains to reach their ideal arrangement at the molecular level. This is typically achieved by maintaining the material at an elevated temperature for a prolonged period.
Unfortunately, perfection requires too much time to be practical from an economic standpoint. So the objective of good process control is to achieve a level of structural stability that is adequate for applications in the real world. When a part is molded in an amorphous polymer, the material has not reached thermodynamic equilibrium. It spends the rest of the life of the product trying to get there. Physical aging can be thought of as the slow contraction of the polymer as the chains get closer, collapsing into the excess free volume that remained when the part was first produced. This closer approach produces a structure that is stronger and stiffer than the original matrix, however it also loses toughness in the process.
The rate at which physical aging occurs is a function of the difference between the application temperature and the glass-transition temperature (Tg) of the polymer. The closer the temperature is to the Tg the more rapidly physical aging occurs. So it is not surprising, in retrospect, that one of the first practical implications of physical aging was observed in an amorphous polymer with a relatively low Tg, PET polyester.
The amorphous PET used to mold preforms and then blow beverage bottles has a Tg of about 172 F (78 C). At room temperature, the degree of physical aging needed to produce a measurable change in mechanical properties can be as long as one to two years. However, when bottles were stored in a warehouse at 120 F (49 C), this reduced the gap between the application temperature and the Tg by about 50% and accelerated the rate of physical aging by approximately an order of magnitude. Bottles stored under these conditions exhibited a measurable loss in impact strength in just a few weeks.
Physical aging only occurs in what material scientists refer to as glasses, which are essentially any amorphous material that exists as a “solid.” All commercial polymers, even semi-crystalline ones like polyethylene and nylon, contain some amorphous regions and exhibit a corresponding glass transition. Therefore, technically it is possible for semi-crystalline materials to undergo physical aging and the associated changes in properties.
However, in most cases these changes are overshadowed by the contributions from the crystalline phase. But some semi-crystalline materials achieve a relatively low level of crystallinity that is highly dependent upon processing conditions. These tend to be the high-performance materials such as PEEK and PPS, where failure to cool the polymer at an appropriate rate will result in an almost completely amorphous structure. In these materials physical aging can occur and has been observed along with the associated property changes.
Because physical aging involves a change in volume, it can be detected as a change in dimensions. This represents a relatively small dimensional change, as a percentage of the size of the part, so detection either requires making very precise measurements or the part must be very large so that this small percentage results in a readily measurable difference. Precise dimensions are not demanded in a beverage bottle, but there are industries that make measurements in microns. They have observed that parts molded in amorphous polymers with low glass-transition temperatures, such as rigid PVC, continue to exhibit a very small degree of dimensional change over a period of months. These changes are much smaller than the ones that occur due to the solid-state crystallization that we discussed previously, but they can still result in a part that drifts out of print over an extended period of time, even though the parts are only exposed to room temperature.
This process can be slowed down if the parts are stored at sub-ambient temperatures, but it cannot be stopped completely unless the temperature is lowered to the point where the polymer undergoes another more subtle transition called the beta transition. For most polymers this transition takes place at a very low temperature that would not be practical for part storage and certainly will not be encountered in most application environments. For example, the beta transition in PVC occurs near -58 F (-50 C) and in polycarbonate it is at -148 F (-100 C).
Up to this point, we have discussed long-term behavior that causes molded parts to become smaller over time. But there are some long-term influences that actually cause parts to grow. In the next installment we will discuss this behavior and look at the mechanisms behind it.
Editor’s note: You can read the next part in the series by clicking here.
Related Content
Prices of All Five Commodity Plastics On the Way Up
Despite earlier anticipated rollover in prices for most of the volume commodity resins, prices were generally on the way up for all going into the third month of first quarter.
Read MoreFirst Quarter Looks Mostly Flat for Resin Prices
Temporary upward blips don't indicate any sustained movement in the near term.
Read MorePrices for PE, PS, PVC, PET Trending Flat; PP to Drop
Despite price increase nominations going into second quarter, it appeared there was potential for generally flat pricing with the exception of a major downward correction for PP.
Read MoreLanxess and DSM Engineering Materials Venture Launched as ‘Envalior’
This new global engineering materials contender combines Lanxess’ high-performance materials business with DSM’s engineering materials business.
Read MoreRead Next
Dimensional Stability After Molding—Part 2
After molding, acetal parts can continue to shrink at room temperature and even in the cold.
Read MoreDimensional Stability After Molding: Part 1
The degree to which molded parts shrink as they cool is largely dependent upon the composition of the material being processed.
Read MoreLead the Conversation, Change the Conversation
Coverage of single-use plastics can be both misleading and demoralizing. Here are 10 tips for changing the perception of the plastics industry at your company and in your community.
Read More
.jpg;width=70;height=70;mode=crop)