Materials: Analyzing Filler Content
The process is considered simple. But things aren’t always as they seem.
Frequently the filler content of a material needs to be measured either for quality control purposes or as part of a troubleshooting exercise where verification of a specification for filler content is needed. For purposes of this article we will limit our discussion to glass content.
Determining glass content in a polymer by thermal methods is relatively simple, since most polymers are carbon-based and therefore will decompose in air at temperatures no higher than 650 C (1202 F). At this temperature the glass is very stable and will maintain its original form, so that any residue that remains at the conclusion of the test can be examined to determine if it is long fiber, short fiber, milled glass, beads, etc.
There are two primary options for making this measurement: ash testing and thermogravimetric analysis (TGA). Ash testing is much simpler and utilizes less expensive instrumententation. It consists of weighing a sample, then placing it in a crucible that goes into a furnace, where it is heated until all the polymer is decomposed. The remaining ash is then weighed and the filler content is calculated.
It is always advisable to examine the residue to ensure that the filler does not contain other constituents. For example, if the sample is white it may contain TiO2, which is also stable at the test temperature and will remain at the conclusion of the test. Many materials may contain only 1-2% white pigment, which will not alter the ash content result appreciably, especially if the glass content is relatively high (30-50%).However, some resins that are dark colored in their natural state, such as polyphenylene sulfide and polyetherimide, may require TiO2 levels as high as 10% to achieve the desired color.
A thermogravimetric analyzer consists of a very sensitive analytical balance, a furnace that can be heated in a programmed manner, and software that continuously monitors the mass of the sample and graphs this as a function of temperature. Where ash testing gives information about the beginning and ending condition of the sample, TGA shows the whole process. Its disadvantage is that it can only work with small samples (10-50 mg), while ash tests use 2-3 g and therefore provide for a more representative sample.
But the ability to graph the weight-loss process is an important part of material identification, since many polymers have distinctive temperature ranges over which they decompose. If the test is started in nitrogen and then is switched to air or oxygen after all the polymer that can be removed in nitrogen is gone, a second data point is produced—the ratio between the weight losses in the two atmospheres.
Different polymers form different amounts of char when heated in nitrogen and then lose this remaining mass when heated in air. Materials like polyethylene and polypropylene produce no char—the entire sample decomposes in nitrogen. ABS and nylon typically produce about 2% of the polymer weight loss as char; polycarbonate gives 25%; and PPS will lose almost exactly the same amount of mass during the second stage of the test in oxygen as it does during the first part of the test that is run in nitrogen.
For polymers that do produce a high level of char, it is essential that the sample be exposed to oxygen or air at a very high temperature in order to remove the char so that it is not considered in the filler calculation. Visually examining the ash at the end of the test is an important step in ensuring complete removal of any char that forms during decomposition in nitrogen. If the residue is black, then oxygen is not being introduced properly and some of the remaining mass is polymer char and not filler. If the presence of polymer char is not considered, all the residue will be reported as ash content.
Recently I read a report on the filler content of a commercial polyamide-imide that is known to contain no inorganic fillers. However, the report showed a “filler content” of nearly 60%. A closer reading showed that the results had been obtained by TGA in nitrogen. Polyamide-imide is a material that produces a very high level of char, and this char was being reported as ash.
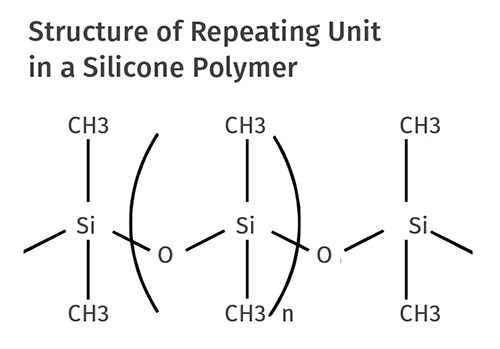
But what if the polymer is not carbon-based but instead silicon-based, like silicone? The backbone of the silicone polymer contains silicon and oxygen but no carbon. However, there are carbon-based groups attached along the side of the polymer backbone that will decompose at high temperatures. Figure 1 shows the chemistry for the repeating unit of what is generically referred to as a silicone polymer. The formal chemical name for the polymer is polydimethyl siloxane. The siloxane term refers to the Si-O linkages that form the backbone and CH3 groups are known as methyl groups. The most-often-used silicones today are elastomers. But silicones have existed as rigid thermosets for many years and they are often highly filled. Therefore, the filler content is likely to be of interest.
The difficulty in determining the filler content of these materials comes in distinguishing between glass fiber in the compound and glass that is actually created as the silicone polymer decomposes. When silicones decompose, only the methyl groups are driven off; the Si-O backbone remains. We can use our knowledge of atomic weights to calculate the ratio of weight lost to weight retained. Silicon has an atomic weight of 28, oxygen is 16, carbon is 12, and hydrogen is 1. By doing some simple math we can see that the total mass of the silicone repeating unit is 74 atomic mass units. Of this amount, 30 atomic mass units (40% of the total) will be lost during decomposition while the other 60% will remain. And because glass is mostly silicon dioxide, the chemical composition of the polymer residue will look like the glass filler. So a TGA or an ash test performed on an unfilled silicone will produce an ash content of 60%; it can never be less than this value.
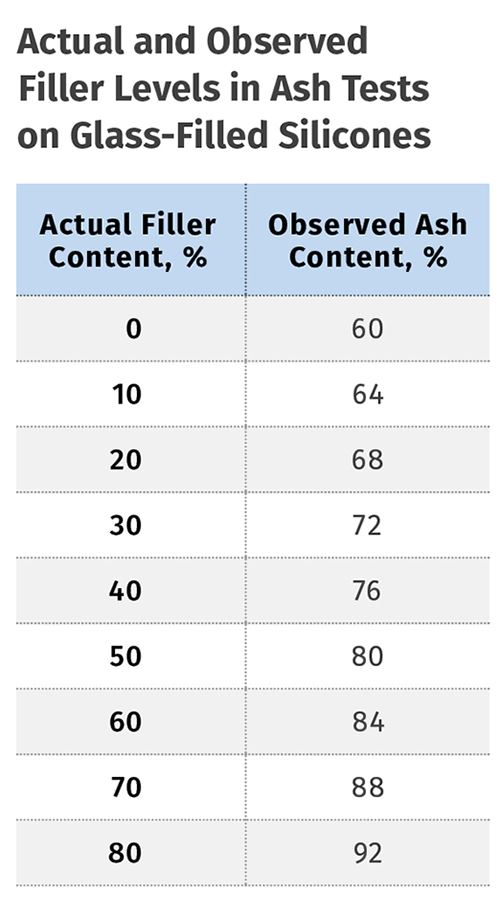
Often I see reports where ash tests or TGA tests have been performed on a material known to be a silicone and I am almost always suspicious of the reported “filler” content because very few analysts consider the contribution of the polymer to the amount of inorganic residue. As an example, a 50% glass-filled silicone will produce an ash content of 80%. The additional 30% comes from the polymer portion that remains along with the glass as a non-combustible residue. The table on p. 28 shows the relationship between the actual filler content and what will be observed when the ash test or TGA is performed. The agreement improves as the amount of actual filler increases, but the two values will never be exactly the same.
We have demonstrated another variation on what is considered a straightforward process of determining the ash content of a material. It proves that some creative thinking needs to go into the analytical process, and shows that sometimes things are not as they seem.
Related Content
Polymer Science for Those Who Work With Plastic — Part 1: The Repeat Unit
What are the basic building blocks of plastics and how do they affect the processing of that material and its potential applications in the real world? Meet the repeat unit.
Read MoreThe Fundamentals of Polyethylene – Part 2: Density and Molecular Weight
PE properties can be adjusted either by changing the molecular weight or by altering the density. While this increases the possible combinations of properties, it also requires that the specification for the material be precise.
Read MorePolyethylene Fundamentals – Part 4: Failed HDPE Case Study
Injection molders of small fuel tanks learned the hard way that a very small difference in density — 0.6% — could make a large difference in PE stress-crack resistance.
Read MoreWhat's the Allowable Moisture Content in Nylons? It Depends: Part 2
Operating within guidelines from material suppliers can produce levels of polymer degradation. Get around it with better control over either the temperature of the melt or the barrel residence time.
Read MoreRead Next
For PLASTICS' CEO Seaholm, NPE to Shine Light on Sustainability Successes
With advocacy, communication and sustainability as three main pillars, Seaholm leads a trade association to NPE that ‘is more active today than we have ever been.’
Read MoreLead the Conversation, Change the Conversation
Coverage of single-use plastics can be both misleading and demoralizing. Here are 10 tips for changing the perception of the plastics industry at your company and in your community.
Read More
.jpg;width=70;height=70;mode=crop)