Fundamentals of Polyethylene – Part 5: Metallocenes
How the development of new catalysts—notably metallocenes—paved the way for the development of material grades never before possible.
In Part 1 of this series, we gave a brief overview of the historical developments related to polyethylene. The most important aspects of the advances made in the synthesis of PE involved the development of new catalysts.
By definition, catalysts are substances that promote a chemical reaction without actually becoming part of the product. An example is the dissociation of hydrogen gas, composed of molecules of two hydrogen atoms, into monatomic hydrogen using a platinum catalyst. The platinum makes the separation of the hydrogen atoms occur much more readily, but the final hydrogen product contains no platinum. (The technical reader will recognize that while in classical chemistry it is true that the catalyst does not become part of the product, in polymerization reactions catalyst residues are often found in the final product). These substances allow the chemical reaction to run more efficiently and, in the case of polymers, to control the structure of the product in ways that were not possible with older technology.
New Catalysts
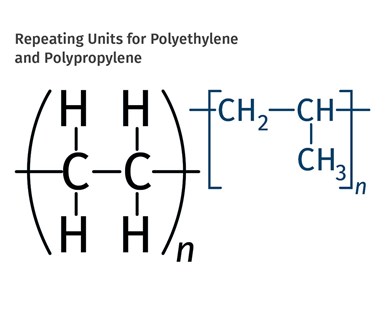
FIG 1 Shown here are the chemical structure of the ethylene and propylene units that make up the polymer chains. If the chain can be made in a linear configuration, multiple chains can pack close together and produce HDPE. In PP, one of the four pendant groups is not a hydrogen atom. Instead it has been replaced by a multi-atom structure known as a methyl group.
New catalysts often bring unforeseen benefits that were not part of the initial development effort. The same Ziegler-Natta and Phillips catalysts that made HDPE possible also ushered in the commercial era of polypropylene. Before those catalysts were developed, the researchers who first developed PE using their high-pressure process naturally sought to extend their work to other substances. Propylene gas was a natural next step. Polymerization of propylene did, in fact, occur in their high-pressure reactor.
But unlike the PE, which was a crystalline solid at room temperature, the PP was a sticky, viscous fluid. While useful as an adhesive, it could not be fabricated into the types of products that polyethylene could be molded into. Using the Ziegler-Natta catalysts, propylene was polymerized into a crystalline solid with properties that extended the capabilities available in polyethylene.
New catalysts often bring unforeseen benefits that were not part of the initial development effort.
The problem with PP can be understood by looking at the chemical structure of the ethylene and propylene units that make up the polymer chains, shown in Fig. 1. Hydrogen atoms are the smallest atoms in existence; therefore, they do not create much space between polymer chains. If the chain can be made in a linear configuration, multiple chains can pack close together and produce what we know as high-density polyethylene (HDPE). In addition, having hydrogen atoms attached to the carbon backbone throughout makes for a very symmetrical arrangement.
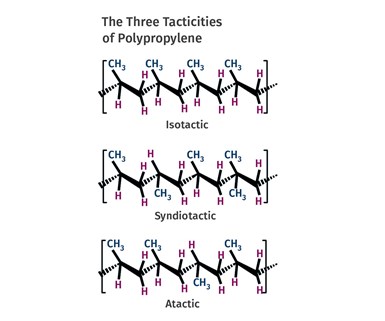
FIG 2 Methyl groups can be arranged in three different patterns. Almost all commercial PPs are primarily isotactic, meaning that the methyl group is located in the same position on each repeating unit.
Metallocene
In polypropylene, one of the four pendant groups is not a hydrogen atom. Instead it has been replaced by a multi-atom structure known as a methyl group (-CH3). This group is bigger and bulkier than a hydrogen atom and it creates more space between the polymer chains. It turns out that the methyl groups can be arranged in three different patterns, as shown in Fig. 2. Almost all commercial polypropylenes are primarily isotactic, meaning that the methyl group is located in the same position on each repeating unit. This allows for sufficient regularity in the structure so that crystals can form and polypropylene in this form actually has a higher melting point than HDPE and is a useful, solid material at room temperature. This is the type of polypropylene that the Ziegler-Natta and Phillips catalysts made possible.
The team that tried to use the brute force of heat and pressure in the 1930s also made PP. But without the advanced catalysts that came along in the 1950s, they could not control the placement of the methyl group. Instead, they produced atactic PP, a molecule where the placement of the methyl group varies in an unpredictable way. With this disordered arrangement, the polymer chains cannot get close enough to each other to form crystals and the material remains amorphous.
If amorphous polymers are to be useful at room temperature, they must have a glass-transition temperature (Tg) that is above room temperature. Unfortunately, the Tg of PP is approximately 0 C (32 F). So, at room temperature, in the absence of a crystalline structure, the material remains a viscous, sticky fluid. Essentially, the Ziegler-Natta and Phillips catalysts, by allowing for a rearrangement of the atoms in polypropylene, converted this glue into a useful solid material.
The metallocene catalysts have made it possible to achieve much narrower distributions than were previously possible.
The metallocene catalysts brought another new feature to the world of PE and PP. To fully appreciate this advancement, it is important to understand that while we sometimes talk about the molecular weight of a polymer as though it were a single value, all commercial polymers consist of chains that vary greatly in length. The molecular weight that we often talk about is really an average of all the contributions from these different chain lengths. This is similar to the situation where a quality-control person evaluates the parts in a capability study. A critical dimension on the part will have a certain average value for the entire sample population, but within that population there will be parts that are larger than the average and others that will be smaller.
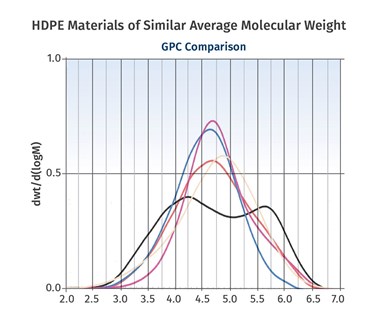
FIG 3 Shown here is a range of HDPEs of comparable average molecular weight that exhibit very different molecular-weight distributions.
Figure 3 shows a range of HDPEs of comparable average molecular weight that exhibit very different molecular-weight distributions. Generally, narrower distributions provide for improved properties. However, broad distributions are associated with easier processing. Metallocene catalysts have made it possible to achieve much narrower distributions than were previously possible. This has created opportunities to make products that were not possible before these catalysts were developed.
In our next and final segment of this series, we will take one last look at the subject of density and molecular weight in HDPE in an application where a change to these two properties is expected to significantly extend the life of a product.
About the Author Mike Sepe is an independent, global materials and processing consultant whose company, Michael P. Sepe, LLC, is based in Sedona, Ariz. He has more than 40 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting, and failure analysis. Contact: (928) 203-0408 • mike@thematerialanalyst.com.
Related Content
The Fundamentals of Polyethylene – Part 1: The Basics
You would think we’d know all there is to know about a material that was commercialized 80 years ago. Not so for polyethylene. Let’s start by brushing up on the basics.
Read MoreThe Fundamentals of Polyethylene – Part 2: Density and Molecular Weight
PE properties can be adjusted either by changing the molecular weight or by altering the density. While this increases the possible combinations of properties, it also requires that the specification for the material be precise.
Read MorePrices Up for PE, PP, PS, Flat for PVC, PET
Trajectory is generally flat-to-down for all commodity resins.
Read MoreFundamentals of Polyethylene – Part 6: PE Performance
Don’t assume you know everything there is to know about PE because it’s been around so long. Here is yet another example of how the performance of PE is influenced by molecular weight and density.
Read MoreRead Next
Beyond Prototypes: 8 Ways the Plastics Industry Is Using 3D Printing
Plastics processors are finding applications for 3D printing around the plant and across the supply chain. Here are 8 examples to look for at NPE2024.
Read MorePeople 4.0 – How to Get Buy-In from Your Staff for Industry 4.0 Systems
Implementing a production monitoring system as the foundation of a ‘smart factory’ is about integrating people with new technology as much as it is about integrating machines and computers. Here are tips from a company that has gone through the process.
Read MoreSee Recyclers Close the Loop on Trade Show Production Scrap at NPE2024
A collaboration between show organizer PLASTICS, recycler CPR and size reduction experts WEIMA and Conair recovered and recycled all production scrap at NPE2024.
Read More
.jpg;width=70;height=70;mode=crop)