What You Can Learn From Pharma Compounders
Twin-screw extrusion has been a standard in plastics compounding for decades but has only recently been embraced by the pharmaceutical industry. Here’s what plastics compounders might learn from the ‘newbies’ to help make a better, more consistent product.
Twin-screw extrusion has been an established industrial technology to manufacture plastics compounds for 50+ years. Prior to the acceptance of twin-screw extruders (TSEs), batch mixers and single-screw extruders were often the mixing devices of choice among compounders. But now, the ability to run many different formulations consistently on the same extruder, in combination with its self-cleaning nature, have made the TSE the machine of choice for plastics compounding.
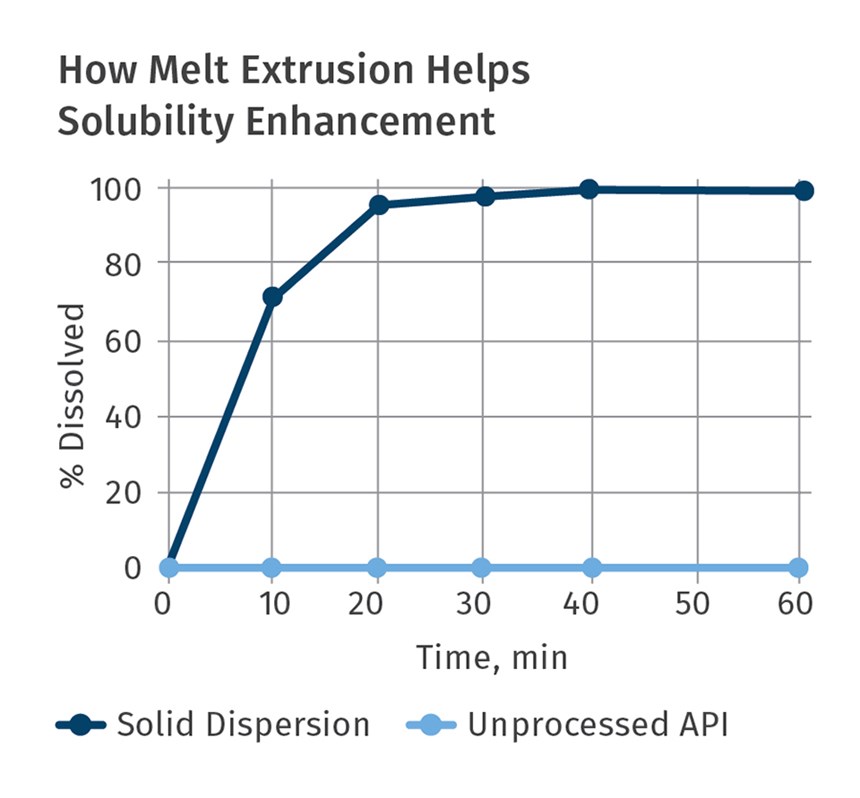
In the last dozen years or so, the pharmaceutical industry has also embraced twin-screw extrusion to manufacture a myriad of dosage forms and multi-functional medical devices. TSEs are now commonly used to mix active pharmaceutical ingredients (APIs) with polymers and additives that serve as binders. Just like in a color masterbatch application, the TSE is utilized as a continuous mixer to make a high-quality, consistent drug-delivery dosage form, including tablets, implantable devices, wound care, and many other products. Applications range from controlled-release systems to oral bioavailability.
Without question, pharmaceutical companies have reaped the benefit of 100 years of TSE technological developments and process refinement emanating from both industrial and research efforts in plastics. But now might be time for plastics companies to follow the lead set by pharma companies and implement—at least some of the time—current Good Manufacturing Practices (cGMP) used in pharma operations to ensure manufacturing efficiencies, in order to make a better plastics product.
Pioneering development activities in the late 1980s and 1990s spawned several commercial drug-delivery compositions using TSEs. Driven by the FDA’s Process Analytical Technology (PAT) initiative, use of twin-screw extruders has since then been embraced by virtually every major worldwide pharmaceutical company.
Questions about compounding? Visit the Compounding Zone to learn more.
Historically, most drug-delivery dosage forms were manufactured via batch processes. The FDA’s 2004 PAT initiative provided drug makers a framework for pharmaceutical development, manufacturing, and quality assurance through in-line monitoring. In a nutshell, the PAT initiative strongly encouraged that new dosage forms be developed and manufactured by continuous processing with in-line monitoring of key parameters. It literally could have been written by an extruder supplier.
Twin-screw extruders process materials in channels bounded by screw flights and barrel walls, and are therefore referred to as small-mass continuous mixers. The motor inputs energy into the process for mixing via the rotating screws. Process control parameters include screw speed (rpm), feed rate, temperatures along the barrel and die, and vacuum level. Typical parameters that are monitored for in-line quality measurement include melt pressure, melt temperature, motor amperage, and in-line IR sensors. Programmable Logic Controllers (PLCs) and touch-screen Human Machine Interfaces (HMIs) are now common, as compared with older discrete controls and readouts.
The basic design of a TSE for plastics or pharmaceutical usage is the same. The mixing mechanisms and staging of unit operations are also virtually identical. TSE process technologies proven in industrial settings can often be quickly implemented for a pharmaceutical process—the TSE process is a known quantity.
TSEs utilize segmented screws that are assembled on high-torque shafts. Barrels are also modular and integrate internal bores for cooling. Segmented screws and barrels, in combination with the controlled pumping and wiping characteristics of co-rotating screws, allow screw/barrel geometries to be matched to the process tasks. The counter-rotating, intermeshing twin-screw mode is also utilized for PVC, masterbatch, and pharmaceutical mixing applications.
The intense mixing associated with the short inter-screw mass-transfer characteristics inherent in a TSE facilitates highly efficient mixing operations, compared with large-mass batch mixers. Entrapped air, moisture, and volatiles are removed by vacuum venting in the extrusion process. The relatively short residence times (10 sec to 2+ min) associated with a TSE are beneficial for many heat- and shear-sensitive materials, as the TSE can be designed to limit exposure to elevated temperatures to just a few seconds.
TSEs are starve-fed, with the output rate determined by the feeder(s), which meter pellets, liquids, powders, and fibers into the process section. The TSE screw rpm is independent from the feed rate and is used to optimize compounding efficiencies. Just like pigments, some APIs require dispersive mixing in the TSE, and some benefit from distributive mixing. For instance, a shear-sensitive API or pigment can be metered in the latter stages of the process section to avoid the high-shear intensity associated with melting.
Co-rotating TSEs currently represent 90% of the TSEs utilized to produce masterbatch products. Interestingly, a higher percentage of counterrotating, intermeshing TSEs are used by pharmaceutical manufacturers, perhaps because there is not a pre-conceived bias in favor of co-rotation.
As an example of a key process parameter to monitor (whether it’s a plastics compound, masterbatch, or drug dosage form) is the Specific Energy (SE) that is being input by the motor into each lb or kg of material being processed. If the SE suddenly changes, this indicates that the equipment, process conditions, or raw materials have changed, and the end product may be different.
SE is calculated as follows:
KW (applied) = kW (motor rating) x % torque x RPM running/
Max. RPM X 0.97 (gearbox efficiency)
Then:
Specific Energy = kW (applied)/(kg/hr)
Units:
SE is denoted in kW per kg/hr
kW = kilowatts (the motor rating in kW = HP x 0.746)
% Torque = % used of the maximum allowable torque
RPM = Screw rotation/min
A strong case could be made that SE should be monitored and logged for any extrusion process, yet oftentimes it isn’t. It’s worth asking why not.
Downstream systems are also similar to plastics for pharmaceutical applications. Strand and die-face pelletization are used for water-soluble pharmaceutical products. Instead of water, the pellets are quenched on conveyors or conveyed and cooled in air to facilitate pellet formation. Smaller pellets can be used for direct capsule filling, whereas larger pellets are typically milled and pressed into tablets. Lamination systems are used for transdermal and dissolvable film applications. Tubing systems produce anti-microbial structures, while coextruded rods are integrated into contraceptive devices (such as the NuvaRing). The applications are endless.
TSEs utilized in cGMP environments generally adhere to FDA part 11 of Title 21 of the Code of Federal Regulations (CFR) regarding electronic records and signatures, which defines the criteria under which electronic records are deemed trustworthy. Practically speaking, Part 11 requires drug makers to implement controls, audits, and system validation for software and systems involved in processing electronic data. Parameters that are being monitored must be verified and periodically calibrated—a good practice for any extrusion installation.
In pharmaceutical compounding, parameters that are being monitored must be verified and periodically calibrated—a good practice for any extrusion installation.
Protocols must be followed to limit system access to authorized individuals and implement operational checks, device checks, controls over systems documentation, and a plethora of other guidelines. Strict adherence to how records are copied and retained is part of the guideline.
Equipment validation documentation related to installations in a pharmaceutical class environment is much more intensive than for a typical plastics machine. Detailed project-specific document packages for the Factory Acceptance Test (FAT), Installation Qualification (IQ), and Operational Qualification (OQ) are required for pharmaceutical-class installations, which typically add months of time and effort to the installation and commissioning of the equipment.
There are also cGMP guidelines for cleaning pharmaceutical class TSE systems. For instance, the equipment must be cleaned at appropriate intervals, following written procedures that must be specific and detailed. After cleaning, equipment must be protected from contamination prior to use and inspected for cleanliness just before using. Records and equipment logs of all cleaning and inspections must be kept, and the time between end-of-processing and cleaning procedures must be recorded. Similar protocols would benefit any plastics processing operation.
Due to the regulatory requirements inherent in manufacturing a drug dosage form, all aspects related to the TSE manufacturing cell necessitate a more regimented and documented approach than is standard practice in the plastics industry.
The suggestion here is not that FDA guidelines and regulations for pharmaceutical products be strictly applied to the manufacture of plastics compounds, but that it may be useful to audit the practices of pharmaceutical manufacturing companies and selectively implement those that are practical and useful to help make a more consistent, repeatable product.
ABOUT THE AUTHOR: Charlie Martin is president and general manager of Leistritz, a leading supplier of twin-screw extrusion equipment for compounding, devolatilization, direct extrusion, pharmaceutical, and other applications. Martin has been in the extrusion industry for more than 25 years, is a member and former chair of the SPE Extrusion Div., and has given 100+ papers at various technical conferences on a range of extrusion topics around the world. Contact: (908) 685-2333; cmartin@leistritz-extrusion.com; leistritz-extrusion.com.
Related Content
Small Batches, Big Success
With no minimum order and an impeccable record of on-time delivery, Precision Color Compounds is becoming a force in the color masterbatch business.
Read MoreSirmax Adapts Integrated Recycling Approach to US Supply Conditions
Integrating compounding and recycling to leverage untapped postindustrial recycling feedstocks.
Read MoreHow to Maintain Pelletizing Quality When Acid Attacks
Developments in the chemistry of polymers and additives have made corrosion a real problem in pelletizers. Here’s how to ward it off.
Read MoreThe Path to Pellet Perfection
In underwater pelletizing, numerous variables in the equipment, process and material affect pellet shape, consistency and quality factors such as fines. Defining the “perfect” pellet depends on the conditions of end use, and achieving that ideal requires understanding of the causes of imperfections.
Read MoreRead Next
Lead the Conversation, Change the Conversation
Coverage of single-use plastics can be both misleading and demoralizing. Here are 10 tips for changing the perception of the plastics industry at your company and in your community.
Read MoreMaking the Circular Economy a Reality
Driven by brand owner demands and new worldwide legislation, the entire supply chain is working toward the shift to circularity, with some evidence the circular economy has already begun.
Read MoreBeyond Prototypes: 8 Ways the Plastics Industry Is Using 3D Printing
Plastics processors are finding applications for 3D printing around the plant and across the supply chain. Here are 8 examples to look for at NPE2024.
Read More