Graham Accelerates Extruder Deliveries as Tubing Processors Ramp Up
Machine builder expediting shipment of Ultra line of extruders as tube makers ramp up production for ventilators resulting from Coronavirus.
The global Coronavirus pandemic crisis has generated a sharp upsurge in production capacity requirements for ventilator tubing, causing Graham Engineering Corp. to speed up processes for delivering extruders to processors needing more equipment to fill orders.
“In the past three weeks we have seen a strong uptick in orders and inquiries from businesses in North America and the Asia-Pacific looking to add capacity for ventilator tubing,” says David Madar, strategic medical market manager for Graham Engineering. “We are responding by validating available inventory, turning quotes instantly, and committing to extruder supply through accelerated delivery. Now more than ever we are here to support the needs of our medical device customers globally, at a time when demands on the medical supply chain have never been greater.”
The expanded production of ventilators is part of the worldwide increase in the manufacture of medical devices to fight COVID-19. Governments and companies are executing elaborate plans to deploy funding and resources for expanding or re-tooling existing operations. Ventilators are essential for supporting patients most severely impacted by the virus.
“In the United States alone, ventilator demand could be as high at ten to fifteen times pre-pandemic levels, significantly outpacing the strategic stockpile and current production capacity,” notes Gina Haines, chief marketing officer of Graham Engineering.
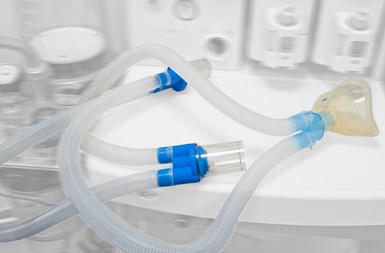
Ventilator tubing kits are supplied to hospitals as single-use breathing circuits. A key component of the circuit is corrugated tubing, typically made of medical grade polyethylene or EVA. In adult kits, the standard inside diameter of the tubing is 22 mm.

Graham Engineering Corporation’s American Kuhne product line is a recognized leader in medical extrusion systems, with a 20-yr history serving the segment and an installed base of over 500 systems at more than 100 customers in 20 countries, the company says. Its systems are designed to extrude tubing and wire jacketing used in medical devices and delivery systems from all medical-grade thermoplastics, include single- and multi-lumen, braided, embedded linear wire, corrugated, and taper tubing.
Related Content
-
Compact Solution for Two-Component Molding
Zahoransky’s new internal mold handling technology foregoes the time, space and money required for core-back, rotary table or index plate technologies for 2K molding.
-
Medical Molder, Moldmaker Embraces Continuous Improvement
True to the adjective in its name, Dynamic Group has been characterized by constant change, activity and progress over its nearly five decades as a medical molder and moldmaker.
-
3D Printed Spine Implants Made From PEEK Now in Production
Medical device manufacturer Curiteva is producing two families of spinal implants using a proprietary process for 3D printing porous polyether ether ketone (PEEK).
















