Unreinforced Amorphous PEI for Medical Devices & Pharmaceutical Applications
SABIC’s new Ultem HU resins help manufacturers choose alternatives to ethylene oxide sterilization.
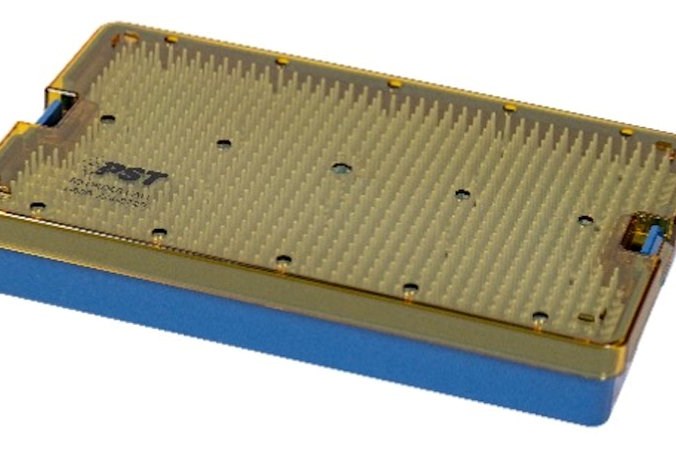
A new family of biocompatible (ISO 10993 or USP Class VI , unreinforced amorphous PEI (polyetherimide) resins from SABIC can help manufacturers of medical devices and pharmaceutical applications choose alternatives to ethylene oxide (EtO) sterilization. This in anticipation of a new ruling by the U.S. Environmental Protection Agency (EPA) that may reduce EtO emissions from commercial sterilization facilities, which has increased demand for materials compatible with other sterilization methods.
Ultem HU resins are stable across many popular sterilization processes including, vaporized hydrogen peroxide (VHP) gas plasma, steam autoclave, gamma radiation, electron beam, X-ray and ultraviolet-C. Moreover, they have been shown to retain their high strength, dimensional stability and attractive aesthetics such as color retention, under exposure to repeated sterilization cycles. This can help reduce breakage and discoloration, thereby potentially extending the useful life of applications made with these materials. According to the U.S. Food and Drug Administration (FDA), approximately 50% of sterile medical devices are treated with EtO, totaling about 20 billion devices each year. EPA’s proposed rule would reduce EtO emissions by an estimated 80 percent.
Related Content
-
Prices Bottom Out for Volume Resins?
Flat-to-down trajectory underway for fourth quarter for commodity resins.
-
Prices of Volume Resins Generally Flat or Lower
Exceptions in early March were PP and PS, which moved up solely due to feedstock constraints, along with slight upward movement in PVC and PET.
-
Soft Prices for Volume Resins
While PP and PE prices may be bottoming out, a downward trajectory was likely for all other volume resins, including engineering types.













