Materials Part 5: Annealing Tips for Crosslinked Polymers
As with semi-crystalline thermoplastics, annealing can be used in thermosetting polymers to obtain a level of crosslinking that may not be possible within the molding cycle.
Just as annealing is used in semi-crystalline thermoplastics to perfect the crystalline structure of the polymer, the same process can be used to obtain a level of crosslinking in thermosetting polymers that may not be achievable within the context of the molding cycle. The property changes that are associated with an increased level of crosslinking are in many ways very similar to those related to an increased degree of crystallinity.
But crystallization and crosslinking, while they respond to the same processing and post-processing influences, are fundamentally different processes. Thermoplastics have been built to a useful chain length before they arrive at the processing plant, and crystallization occurs spontaneously from the melt as temperature declines. At some point in the cooling process, we observe a sharp transition in the material structure that is a function of the chemistry of the material and the ambient or applied pressure.
Once that critical point is attained, the crystallization process continues, provided that the material remains above the glass-transition temperature. That temperature (Tg) is essentially a constant for any given polymer, as long as the molecular weight is high enough to be associated with useful mechanical properties, so the annealing conditions needed to promote additional crystallization are predictable.
Crosslinked materials arrive at the processing plant as a work in process. The chemistry of the material has been established through a chemical reaction that has been stopped before polymerization can truly begin, a state often referred to as “prepolymer.” This material is capable of undergoing further reactions to create the fully developed polymer. These reactions are promoted by elevated temperatures and rely on the presence of reactive groups that are part of the prepolymer, and a catalyst.
Phenolic, the first truly synthetic polymer, is a well-known member of this material family. This material starts from a reaction of phenol with formaldehyde. As the initial stages of the reaction take place, the product increases in viscosity and may at some point become a sticky, viscous material that can be useful as an adhesive. If the process is continued, the material can become a solid with a relatively low melting point. It can then be pulverized and combined with catalyst and the appropriate fillers, at which point it has become a molding resin.
In this form the material has a low melting or softening temperature and an even lower Tg. However, when this material is subjected to elevated temperatures, which are usually provided by a heated mold, a chemical reaction continues the polymerization process, increasing the molecular weight of the polymer by forming crosslinks between the already formed chains, as well as extending those chains. This is a very oversimplified description of polymerization in a thermosetting material.
But of primary concern for this discussion is the fact that in the process of forming the part we are also creating the finished material. The properties of the part will depend to a significant degree upon the degree of crosslinking that is established and this, in turn, is determined by the mold temperature and time that the part is in the mold. Ideally, the part that emerges from the mold is composed of a material with a high Tg that is related to the degree of crosslinking.
But just as molders may not achieve all of the desired crystallinity in a semi-crystalline thermoplastic, they may also not attain all of the desired crosslinking in a thermosetting polymer within the allotted cycle time. In those cases, annealing is performed to enhance the degree of crosslinking. In the parlance of the industry, this is often referred to as post-baking. The idea behind post-baking is to drive the degree of crosslinking to a higher level without extending the molding cycle time or resorting to higher mold temperatures. It is particularly useful in polymers such as phenolics and polyimides that crosslink through a process known as a condensation mechanism. These types of materials have the ability to undergo additional crosslinking to a significant degree under the influence of the elevated temperature associated with post-baking.
The idea behind post-baking is to drive the degree of crosslinking to a higher level without extending the molding cycle time or resorting to higher mold temperatures.
The benefits of post-baking to achieve a higher degree of crosslinking in thermosetting polymers are similar to those obtained through annealing semi-crystalline thermoplastics. Mechanical strength and modulus increase, and with those changes come improvements in creep and fatigue resistance. Dimensional stability at elevated temperatures will also be enhanced, while ductility will decline. And just as there can be problems with dimensional changes during annealing of semi-crystalline thermoplastics, the same issues can occur with post-baking.
In the case of semi-crystalline thermoplastics, we touched on the fact that if too little crystallinity was obtained during the molding process, the attempt to make up the difference with annealing can result in unmanageable problems with shrinkage and warpage. In some crosslinked materials, an additional problem that can arise is blistering of the part. This is caused by volatile byproducts that are naturally produced during condensation polymerization reactions. In the case of post-baking phenolic, the compound that is given off is ammonia. If the ammonia cannot diffuse rapidly enough through the wall of the part, it will produce a distortion in the part.
The time required for post-baking will depend upon the objective. Unlike the process of annealing semi-crystalline thermoplastics, one of the important consequences of post-baking a crosslinked material is an increase in the Tg. This increase is dependent upon both time and temperature, and the relationship is non-linear. So, it is important to understand the material, the state it is in when it comes out of the mold, and the performance needed for the application. Another key difference between annealing in the crystals in a semi-crystalline polymer and increasing the crosslink density of thermoset polymers is that in semi-crystalline thermoplastics the annealing temperature must exceed the Tg of the polymer. This is not necessarily the case in thermosets. A phenolic resin with an as-molded Tg of 175 C can be post-baked at 160 C and the Tg will increase.
One of the important consequences of post-baking a crosslinked material is an increase in the glass-transition temperature.
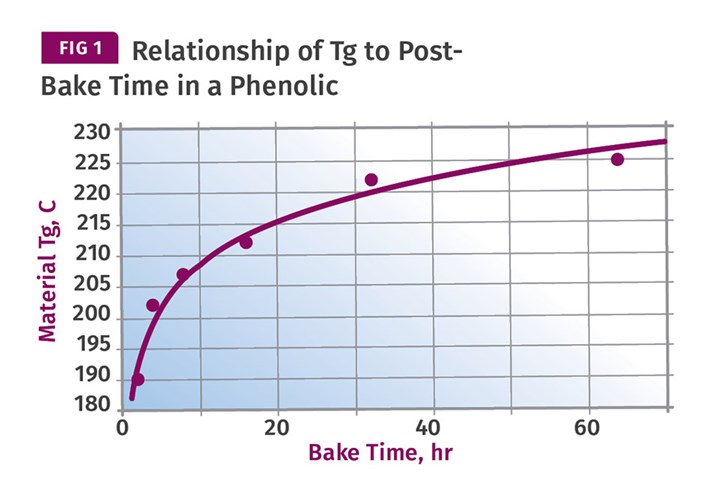
Figure 1 shows the relationship between time and the rise in Tg in a phenolic material, from work done by Ted Morrison at Plenco. This shows that an increase in Tg of approximately 30° C can be attained in about 18 hr of post-baking. But an additional increase of the same magnitude will require 146 hr following the model established in the graph. A higher post-bake temperature can be used, but this risks problems with blistering and warpage.
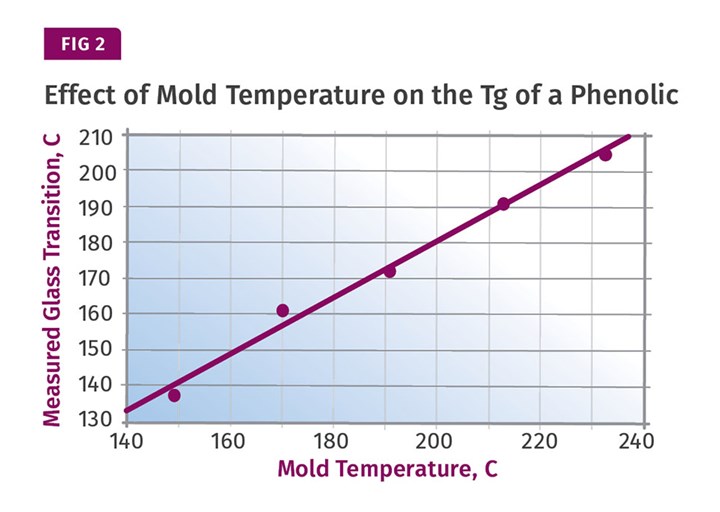
The alternative, as is the case in all of the materials we have discussed to this point, is to develop more structure in the part during the molding process by using a higher mold temperature. Figure 2 shows another result from Morrison’s study that makes the connection between mold temperature and the Tg of the polymer in the part. It should be obvious that with a higher mold temperature, there will be less work to do in post-baking to achieve the desired level of performance.
In our next column we will review annealing practices in thermoplastic polyurethanes, where some remarkable benefits can be achieved in a relatively short period of time.
ABOUT THE AUTHOR: Mike Sepe is an independent, global materials and processing consultant whose company, Michael P. Sepe, LLC, is based in Sedona, Ariz. He has more than 40 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting, and failure analysis. Contact: (928) 203-0408 • mike@thematerialanalyst.com.
Related Content
Prices for PE, PS, PVC, PET Trending Flat; PP to Drop
Despite price increase nominations going into second quarter, it appeared there was potential for generally flat pricing with the exception of a major downward correction for PP.
Read MoreCommodity Resin Prices Flat to Lower
Major price correction looms for PP, and lower prices are projected for PE, PS, PVC and PET.
Read MorePrices Up for PE, ABS, PC, Nylons 6 and 66; Down for PP, PET and Flat for PS and PVC
Second quarter started with price hikes in PE and the four volume engineering resins, but relatively stable pricing was largely expected by the quarter’s end.
Read MoreAdvanced Biobased Materials Company PlantSwitch Gets Support for Commercialization
With participation from venture investment firm NexPoint Capital, PlantSwitch closes it $8M bridge financing round.
Read MoreRead Next
Part 3 Materials: Annealing Tips for Semicrystalline Polymers
For these polymers, annealing is done to establish a level of crystallinity that cannot be practically obtained within the parameters of a normal molding cycle. Here’s some guidance on setting annealing time and temperature.
Read MoreMaterials: Annealing Tips for Amorphous Polymers, Part 2
In amorphous polymers, annealing is performed to draw down the internal stresses to a level not achievable within the conditions of a normal molding process. But a few parameters are important to achieving the desired results.
Read MoreMaterials: Annealing Tips for Semi-Crystalline Polymers: Part 4
You can forgo the elevated mold temperatures normally recommended for high-performance semi-crystalline materials. But it’s risky and likely to yield parts that under-perform expectations … assuming that they emerge from the annealing process looking anything like the drawing.
Read More
.jpg;width=70;height=70;mode=crop)