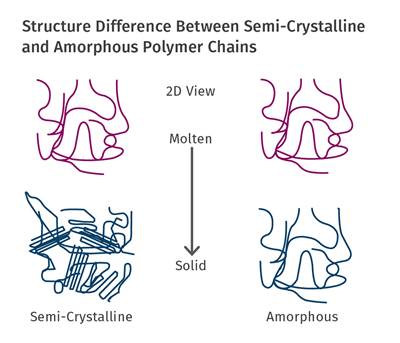
Part 3 Materials: Annealing Tips for Semicrystalline Polymers
For these polymers, annealing is done to establish a level of crystallinity that cannot be practically obtained within the parameters of a normal molding cycle. Here’s some guidance on setting annealing time and temperature.
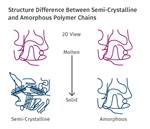
Annealing of amorphous polymers is typically performed to reduce the internal stress in a part below the levels achievable during the molding process. However, in semicrystalline polymers the objective of annealing is to establish a level of crystallinity that cannot be practically obtained within the parameters of a normal molding cycle.
Each semicrystalline polymer has the ability to crystallize to a certain extent that depends upon the chemical structure of the polymer chain. HDPE has a flexible, streamlined chain that allows for efficient crystallization to a very high percentage, while a material like PEEK attains a modest level of crystallinity even under the most carefully controlled process conditions.
Optimum levels of crystallinity enhance a wide range of properties that include strength, modulus, creep and fatigue resistance, and dimensional stability. This last property is very important in applications where very tight tolerances must be maintained in parts that will be used at elevated temperatures. Crystallization is controlled by cooling rate and occurs at a rapid rate during the fabrication process. To achieve what is considered to be an optimal level of crystallization, the temperature of the mold must be maintained above the glass-transition temperature of the polymer. This promotes a level of molecular mobility that allows crystals to form.
Crystallization can only occur in the temperature window below the crystalline melting point and above the glass-transition temperature (Tg). Consider PPS as an example. The melting point of PPS is 280 C (536 F) while the Tg is approximately 130 C (266 F) when determined from a particular dynamic mechanical property. Therefore, the guideline for setting the mold temperature to ensure proper crystallization is a minimum of 135 C (275 F). Processors that pay attention to this requirement will typically select mold temperatures of 135-150 C (275-302 F). But even when this parameter is properly controlled, the relatively rapid rate of cooling involved in melt processing and the limited time that the part spends in the mold will limit the achievement of the crystalline structure to about 90% of what is theoretically obtainable.
One determining factor in selecting an annealing temperature is the maximum temperature to which the part will be exposed in application.
We know that the rate of crystallization is not constant across the entire temperature range between Tg and Tm (melting point. In many polymers, crystals form most quickly at a temperature approximately midway between these two extremes. Therefore, to achieve the most efficient rate of crystallization in PPS, we would use a mold temperature of 205 C (401 F). This is a more challenging mold temperature to maintain, and the difference in mechanical properties between a part produced at this higher mold temperature and one produced at the lower mold temperature is relatively small. Therefore, the typical practice is to use the lower mold temperature.
However, if the molded part will need to operate at 200 C, exposure to this application temperature will produce additional crystallization while the product is in use. We know that as materials crystallize, they shrink. So, a part that goes into the field molded to the proper dimensions and is then exposed to very high application temperatures may change size while in use. If this dimensional change creates a functional problem for the product, then it is necessary to stabilize the dimensions of the part before it goes into use. This is done through annealing.

In amorphous polymers the annealing temperature needs to approach the Tg of the polymer. However, to produce the desired result when annealing a semicrystalline material, the annealing temperature must exceed the Tg of the polymer. The time required will depend upon the part wall thickness, as is the case for amorphous polymers. But the other factor that influences the required time will be the annealing temperature.
As mentioned above, the target annealing temperature is often the midpoint between Tg and Tm. Lower temperatures will require a longer annealing time. Another determining factor in selecting an annealing temperature is the maximum temperature to which the part will be exposed in application. If a part is annealed at 200 C but is then used at 225 C, new crystals will form at the higher use temperature that were not formed during the annealing process. This will produce additional dimensional changes that may be problematic. Therefore, the annealing temperature should be equal to or slightly greater than the maximum temperature at which the part will be used. Just as amorphous polymers cannot withstand annealing temperatures above their Tg, semicrystalline polymers cannot be annealed at temperatures that exceed their crystalline melting point.
Annealing time is best established experimentally for a particular part geometry.
Annealing time is best established experimentally for a particular part geometry. In amorphous polymers, the test used to establish that the objective of annealing has been met is the solvent test that measures residual stress in the part. In semicrystalline resins the benchmark is dimensional stability. A properly annealed part molded in a semicrystalline material should be able to withstand exposure to a time-temperature routine representative of a worst-case application environment without exhibiting an additional change in dimensions.
A good example of this principle can be illustrated for parts designed for exposure to a temperature of 85 C (185 F) for periods of up to 8 hr. An assembly produced from two component parts that had each been annealed at 70 C (158 F) for 1 hr exhibited dimensional changes upon exposure to the application conditions. These changes caused the parts to bind when the assembly was operated, making it non-functional. Annealing at 110 C for the same 1-hr period resulted in assemblies that displayed no change in function after exposure to the application environment.
There is another reason for selecting an annealing temperature that exceeds the highest anticipated use temperature. Crystals that are formed while a material is in the solid state are not as large or as perfect as those that form as the material cools from the melt. Consequently, they do not have the same properties and they do not impart the same benefits to the overall structure of the material. Specifically, crystals that are formed at a particular annealing temperature will melt at a temperature just a few degrees above the temperature at which they were produced. Therefore, crystals that are produced at a temperature below the maximum use temperature of the part will not survive that exposure and are not useful.
Because additional shrinkage during annealing of a semicrystalline material is inevitable, the dimensions of the as-molded part must be larger than the final target dimensions. This may require that parts be molded out of print so that they can meet the print once they have gone through the annealing process. It is important, therefore, that a relationship be established between the as-molded dimensions and the annealed dimensions.
Annealing temperatures for many semicrystalline polymers are high enough to produce other effects on the polymer that are potentially damaging. For example, the midpoint between the Tg and the Tm of nylon 66 is 160 C (320 F). At this temperature nylon can rapidly oxidize. This can cause a change in the color of the material, but more importantly it can result in a permanent loss in mechanical properties, particularly those associated with ductility. Consequently, for materials like nylons annealing is best performed either in an inert atmosphere, under vacuum, or in a fluid that will act as an oxygen barrier and will not alter the properties of the material. For example, nylon parts can be annealed in hot mineral oil to prevent oxidation and improve heat transfer. Because mineral oil is nonpolar, the nylon will not absorb the oil and no plasticizing effects will be observed.
Annealing in semicrystalline materials is ideally done in order to perfect the structure of a part that has already been molded according to optimal procedures. However, some processors use the annealing strategy to avoid the demands of the high mold temperatures needed to properly crystallize high-performance materials such as PPS, PEEK, and PPA. This can bring about serious deficiencies in part performance and significant difficulties with process control. In our next article we will look at these problems more closely.
ABOUT THE AUTHOR: Mike Sepe is an independent, global materials and processing consultant whose company, Michael P. Sepe, LLC, is based in Sedona, Ariz. He has more than 40 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting, and failure analysis. Contact: (928) 203-0408 • mike@thematerialanalyst.com.
Related Content
First Quarter Looks Mostly Flat for Resin Prices
Temporary upward blips don't indicate any sustained movement in the near term.
Read MoreThe Effects of Stress on Polymers
Previously we have discussed the effects of temperature and time on the long-term behavior of polymers. Now let's take a look at stress.
Read MorePrices Up for All Volume Resins
First quarter was ending up with upward pricing, primarily due to higher feedstock costs and not supply/demand fundamentals.
Read MoreThe Effects of Time on Polymers
Last month we briefly discussed the influence of temperature on the mechanical properties of polymers and reviewed some of the structural considerations that govern these effects.
Read MoreRead Next
Injection Molding: Melting Amorphous vs. Semi-Crystalline Plastics
Understanding the differences in how each melts is crucial to obtaining melt uniformity.
Read MoreMaterials: Annealing Tips for Amorphous Polymers, Part 2
In amorphous polymers, annealing is performed to draw down the internal stresses to a level not achievable within the conditions of a normal molding process. But a few parameters are important to achieving the desired results.
Read MoreMaterials Part 1: What Annealing Can Do for Your Process
Relatively rapid cooling rates in processing introduce internal stress. If functional problems in use result, annealing may draw down the stress to levels that may not be achievable during processing.
Read More
.jpg;width=70;height=70;mode=crop)