Operating an industrial water chiller incorporates three major concepts: heat transfer, fluid circulation, and refrigeration.
Heat Transfer
Heat always flows from a higher temperature substance to a lower temperature substance.
Heat transfer is the process of moving heat energy from a substance with high heat energy to another substance with lower heat energy. In the case of the chiller, temperature is the measure of energy and heat is the energy itself. Heat energy cannot be destroyed but can be transferred. Heat always flows from a higher temperature substance to a lower temperature substance. This rate of heat flow is commonly expressed in terms of Btu/hr—the quantity of heat, in Btu’s, that flows from one substance to another over a period of one hour.
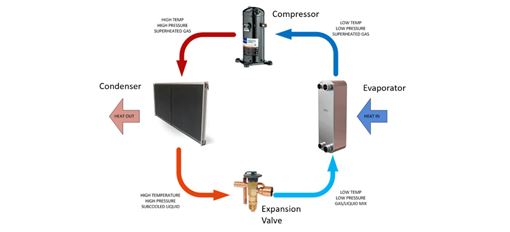
Industrial water chiller operation utilizes heat transfer in two key places: the evaporator and the condenser. In the evaporator, heat transfers from the process recirculating fluid (higher temperature) into the refrigerant (lower temperature). The condenser transfers this heat from the refrigerant (higher temperature) to the cooling source (air or water) at a lower temperature. Each is a part of the refrigeration cycle which will be explained in more detail below.
Fluid Circulation
Industrial water chillers use fluid circulation to deliver the heat from the process to the chiller. This fluid is typically water or a water/glycol mixture. The fluid removes heat from the process, returns to the chiller, transfers heat to the refrigerant through the evaporator, and exits the chiller cold to return to the process.
Refrigeration
Refrigeration is a thermodynamic cycle. Chillers use refrigeration to extract heat from the process circulation fluid and then ultimately reject it to the atmosphere. This system uses a chemical compound called a refrigerant. There are many types of refrigerants and applications depending on the temperatures required, but they all work on the basic principle of compression and phase-change of the refrigerant from a liquid to a gas and back to a liquid. This process of heating and cooling the refrigerant and changing it from a gas to a liquid and back again is the refrigeration cycle. Changing the physical state of a compound (for example, from gas to liquid) is an extremely efficient means to absorb or expel energy.