Melt Flow Rate Testing—Part 6
Once degradation has been established, the discussion inevitably turns to how it happened. It might be expected that the answer to this question is widely known. Not so. The good news is that the influences that cause polymer degradation during processing are few.
While last month’s article was being written, tests were being run on two sets of polycarbonate parts. The results demonstrated exactly the situation that we outlined in that article. Both the “good” and “bad” parts exhibited an excessive increase in melt flow rate (MFR) that is typically an indicator of polymer degradation. The “good” parts were judged to be good because they had performed well in the application. The “bad” parts showed obvious cracking that alerted the end user to the problem.
The molder had not provided a sample of the raw material but it had provided documentation showing the specific grade of material that was used in both runs. The raw material had a nominal MFR of 8.5 g/10 min. The “good” parts gave a MFR of 22.3 g/10 min, or an increase of 159%, while the cracked parts produced a melt flow rate of 66.4 g/10 min, an increase of nearly 670%. These results both exceed the benchmark boundary of 40% MFR increase during processing, but obviously the cracked parts were molded from a material that had undergone a lot more abuse.
When presented with results like this, it is easy to point the finger at the molding process. This assumes that the raw material used to produce the parts was, in fact, as represented. It also assumes that the material was made to the published specification. The value of 8.5 g/10 min represents the published nominal value, not necessarily the value for the lot of material in question. However, if made properly, a grade of material with a nominal value of 8.5 g/10 min. should not exceed 10.5 g/10 min. This still places both of these results well outside the appropriate range.
Once degradation has been established, the discussion inevitably turns to how it happened. It might be expected that the answer to this question is widely known. But the fact that it is asked as often as it is suggests otherwise. The good news is that the influences that cause polymer degradation during processing are few.
The universal concern for all polymers is the combined effect of heat and time while the material is in the melt state. Polymers are typically organic and they have a limited tolerance for the temperatures and shear stresses associated with molding processes, particularly injection molding. Polymers like polyethylene and polypropylene will withstand the processing environment quite well as long as the antioxidant package in the material holds out.
However, polymers such as polyesters and nylons are more susceptible to thermal degradation. The rate at which this degradation occurs depends upon the actual melt temperature of the material in the injection cylinder and the time that it spends there, also known as the residence time.
If you ask material suppliers about the residence time they prefer for their products, you will get answers that typically fall in the range of 5-10 min, depending upon the polymer. Unfortunately, because molds often run in machines with barrels that are larger than would be considered optimal, residence times often exceed these recommended values considerably.
The longest residence time I have personally witnessed is 51 min, and I have heard of a situation where the residence time exceeded 60 min. At these time frames even melt temperatures that remain well within the supplier’s recommended range may not ensure the survival of the material.
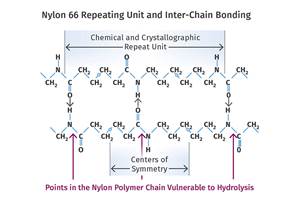
The other major culprit is moisture. This is not a universal concern. Some materials such as polyethylene and polystyrene are non-polar and cannot absorb an appreciable amount of water. Other materials like ABS and acrylic are hygroscopic, but the water will only produce cosmetic problems, it will not chemically attack the polymer. However, a small class of polymers that includes polyesters, polycarbonate, and nylons actually react with excess moisture in a way that breaks chemical bonds within the polymer chain, thus reducing the molecular weight of the polymer.
This process is known as hydrolysis and it can only be prevented by drying the raw material to an appropriate level before the material is introduced into the heating cylinder of the molding machine. For some materials the maximum allowable moisture content for processing is as low as 200 parts per million (0.020%), and optimal levels can be as low as 50 ppm.
As with most chemical reactions, hydrolysis occurs more rapidly at higher temperatures. Therefore, in a situation where melt temperatures are elevated or residence times are extended, improperly dried material will degrade even more quickly than it would under optimal processing conditions. Many processors and end users assume that thermal and hydrolytic degradation will be visually evident. However, this frequently is not the case.
Thermal degradation may result in some discoloration, but colorants can mask this effect, and dark colors such as brown and black can make color shifts undetectable. Hydrolytic degradation is often associated with a visual defect known as splay or silver streaking. But by the time splay appears on the part surface it is already too late. Splay is often caused by volatiles produced when excess moisture in the material boils. The moisture that causes the damage to the polymer molecule has been consumed in the chemical degradation reaction and will not produce a visual defect.
PET is an excellent example of this. PET can be molded with a substantial amount of excess moisture and still produce parts with a good surface appearance because it reacts with the water very efficiently. An alert processor may notice a reduction in the viscosity of the material and the quality-control people performing mechanical tests will certainly observe a decline in mechanical strength and ductility, but the parts will look fine.
There is one more factor that can influence the molecular weight of a polymer during processing and that is the very drying process used to remove the moisture from the material. Since drying involves elevated temperatures, extended drying times can potentially influence the integrity of the material, particularly if the drying temperatures exceed supplier recommendations. This is unlikely to occur in most amorphous polymers like polycarbonate, ABS, and acrylic, because the recommended drying temperatures are usually just 20-30° F below the softening point. Any significant excursion in the drying temperature will quickly produce a sintered mass of material that will no longer flow through the hopper.
However, in semi-crystalline polymers, the recommended drying temperature may be 200-300° F below the melting point. In these cases, significantly elevated drying temperatures can occur without producing any noticeable problems in conveying resin through the material-handling system. But studies have shown that nylon— particularly those grades that are not heat stabilized—will undergo an increase in MFR if dried for extended times at temperatures that exceed supplier recommendations.
These MFR changes occur even before the material is introduced into the heating cylinder of the molding machine. The resulting brittle condition of parts made from this degraded material is often referred to as “overdrying.” However, it has nothing to do with the actual moisture content of the pellets. It is attributable to oxidation, another chemical reaction that results in a reduction in the molecular weight of the polymer.
Unfortunately, this factor has received far too much attention. It is a real concern with nylons, and studies have shown that the oxidation process is driven by drying temperature much more than by drying time. In most polymers, oxidation occurs at a much slower rate than in nylons and can take days or even weeks, particularly if proper temperature control is in place.
But this has not stopped material suppliers from including maximum drying-time recommendations in their literature. Some of these recommended times are remarkably short, as little as 8-12 hr, and they send an unfortunate message to processors that damage to the polymer is likely if these drying times are exceeded.
There is no science behind these relatively new recommendations. When it comes to preserving the molecular weight of a polymer, the risks of not drying enough far outweigh those associated with drying for a longer period of time. In fact, differences in the effectiveness of drying distinguished the “good” from the “bad” polycarbonate parts mentioned at the beginning of this article. While the “good” parts could certainly have been better, they were at least dried to a level that allowed for a functional part. The “bad” parts were not, as evidenced by splay on some of those parts.
Our next article will look at the limitations of the MFR test in detecting resin degradation and why it does not always work.
About the Author
Michael Sepe is an independent materials and processing consultant based in Sedona, Ariz. with clients throughout North America, Europe, and Asia. He has more than 35 years of experience in the plastics industry and assists clients with material selection, designing for manufacturability, process optimization, troubleshooting, and failure analysis. Contact: (928) 203-0408 • mike@thematerialanalyst.com.
Related Content
What is the Allowable Moisture Content in Nylons? It Depends (Part 1)
A lot of the nylon that is processed is filled or reinforced, but the data sheets generally don’t account for this, making drying recommendations confusing. Here’s what you need to know.
Read MoreLet's Take a Journey into the World of Molding Thermosets – Part 1
There are many fundamental differences between thermosets and thermoplastics, from the way raw materials are furnished to the molder and the process in which parts are molded.
Read MorePolymer Science for Those Who Work With Plastic — Part 1: The Repeat Unit
What are the basic building blocks of plastics and how do they affect the processing of that material and its potential applications in the real world? Meet the repeat unit.
Read MoreFundamentals of Polyethylene – Part 5: Metallocenes
How the development of new catalysts—notably metallocenes—paved the way for the development of material grades never before possible.
Read MoreRead Next
Melt Flow Rate Testing–Part 1
Though often criticized, MFR is a very good gauge of the relative average molecular weight of the polymer. Since molecular weight (MW) is the driving force behind performance in polymers, it turns out to be a very useful number.
Read MoreMelt Flow Rate Testing – Part 2
To fully appreciate the strengths and weaknesses of the melt-flow-rate (MFR) test it is important to know something about the way the test is performed.
Read MoreMelt Flow Rate Testing—Part 3
There is a well-established relationship between something called the weight-average molecular weight of a polymer and a parameter known as the zero-shear viscosity.
Read More
.jpg;width=70;height=70;mode=crop)